NCERT Exemplar Class 11 Physics Chapter 11 Thermodynamics are part of NCERT Exemplar Class 11 Physics. Here we have given NCERT Exemplar Class 11 Physics Chapter 11 Thermodynamics.
NCERT Exemplar Class 11 Physics Chapter 11 Thermodynamics
Multiple Choice Questions
Q1. An ideal gas undergoes four different processes from the same initial state (figure). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of 1, 2, 3 and 4 which one is adiabatic?
(a) 4
(b) 3
(c) 2
(d) 1
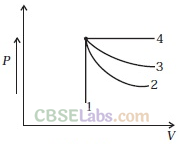
Solution:

Q2. If an average person jogs, he produces 14.5 x 103 cal/min. This is removed by the evaporation of sweat. The amount of sweat evaporated per minute (assuming 1 kg requires 580 x 103 cal for evaporation) is
(a) 0.25 kg (b) 2.25 kg (c) 0.05 kg (d) 0.20 kg
Sol:(a) Rate of bum calories is equivalent to sweat produced. Then, Amount of sweat evaporated/minute

Q3. Consider P-Vdiagram for an ideal gas is shown in figure. Out of the following diagrams, which figure represents the T-P diagram?
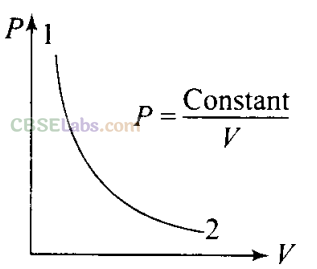
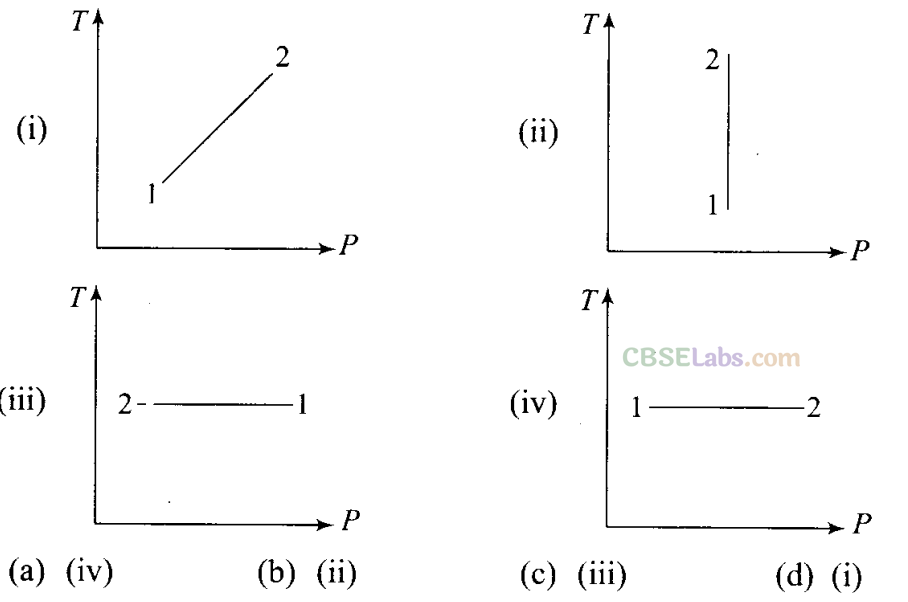
i.e., V 1/p or PV = Constant
Hence, we can say that the gas is going through an isothermal process. Clearly, from the graph that between process 1 and 2 temperature is constant and the gas expands and pressure decreases, i.e., P2<Pl. So, we have to keep in mind while drawing the T-P graph, that temperature (T) is constant and pressure at point 2 is greater than the pressure at 1, which corresponds to diagram (iii).
Q4. An ideal gas undergoes cyclic process ABCDA as shown in given P-V diagram. The amount of work done by the gas is
(a) 6PgV0
(b) -2P0V0
(c) +2 P0Vo
(d) +4Po V0
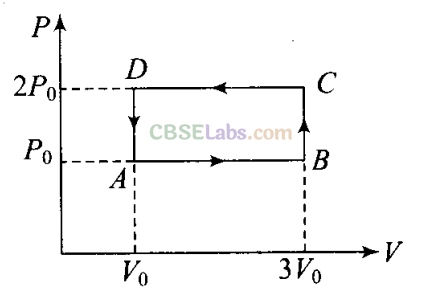
Sol:

Important point: In a cyclic process work done is
1. positive if the cycle is clockwise.
2. negative if the cycle is anticlockwise.
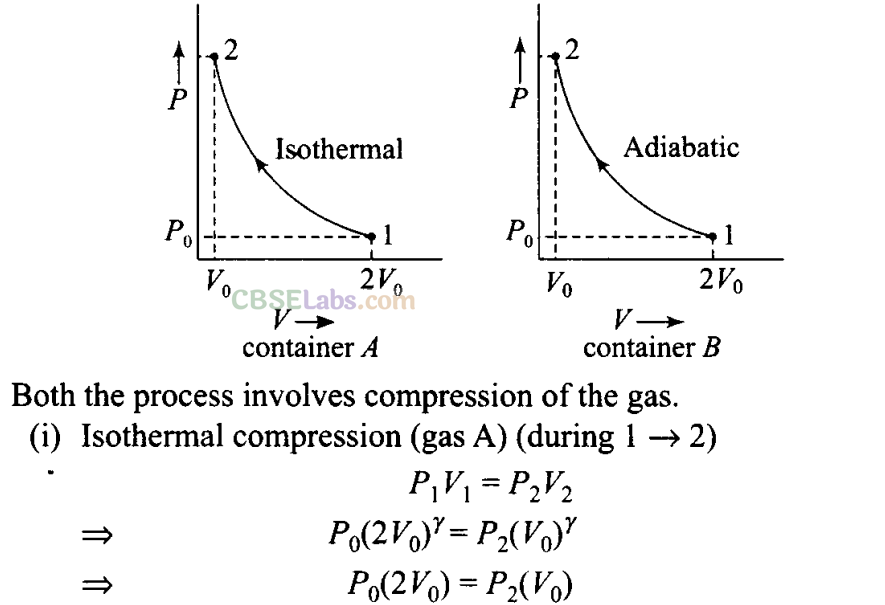
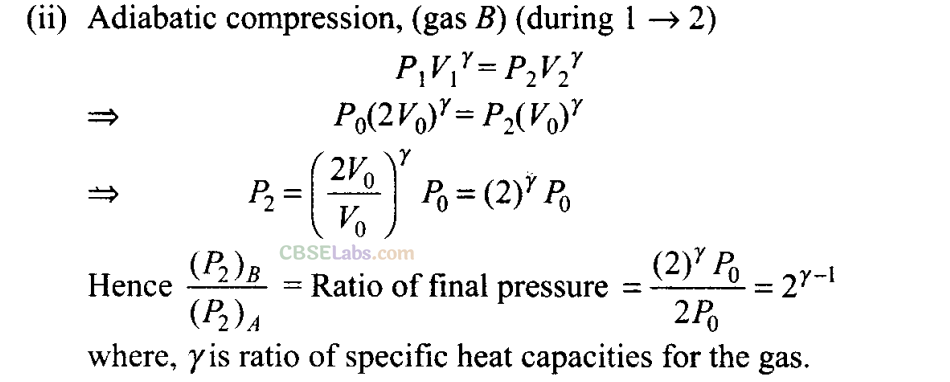
Q5. Consider two containers A and B containing identical gases at the same pressure, volume and temperature. The gas in container A is compressed to half of its original volume isothermally while the gas in container B is compressed to half of its original value adiabatically. The ratio of final pressure of gas in B to that of gas in A is
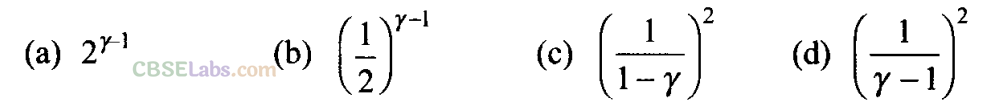
Sol: (a) According to the P-V diagram shown for the container A (which is going through isothermal process) and for container B (which is going through adiabatic process).
Q6. Three copper blocks of masses M1 M2 and M3 kg respectively are brought into thermal contact till they reach equilibrium. Before contact, they were at T1, T2, T3 (T1 > T2> T3). Assuming there is no heat loss to the surroundings, the equilibrium temperature T is (s is specific heat of copper)
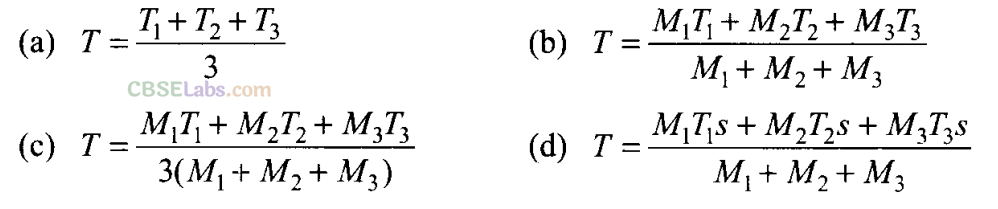
Sol: (b) According to question, since there is no net loss to the surroundings and the equilibrium temperature of the system is T.
Let us assume that Tl,T2< T< T3.
Heat lost by M3 = Heat gained by M1+ Heat gained by M2
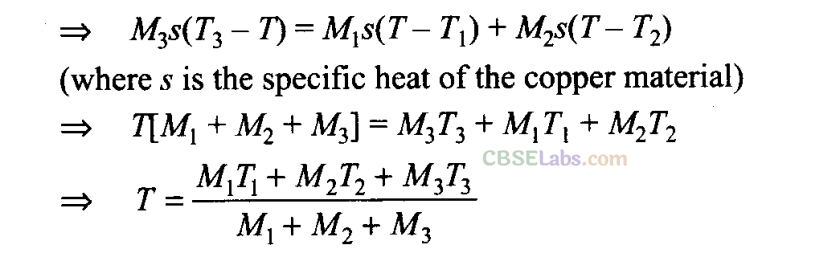
More Than One Correct Answer Type
Q7. Which of the processes described below are irreversible?
(a) The increase in temperature of an iron rod by hammering it.
(b) A gas in a small container at a temperature T1, is brought in contact with a big reservoir at a higher temperature T2 which increases the temperature of the gas.
(c) A quasi-static isothermal expansion of an ideal gas in cylinder fitted with a frictionless piston.
(d) An ideal gas is enclosed in a piston cylinder arrangement with adiabatic walls. A weight w is added to the piston, resulting in compression of gas.
Sol: (a, b, d)
Key concept: Reversible process: A reversible process is one which can be reversed in such a way that all changes occurring in the direct process are exactly repeated in the opposite order and inverse sense and no change is left in any of the bodies taking part in the process or in the surroundings.
The conditions for reversibility are:
• There must be complete absence of dissipative forces such as friction, viscosity, electric resistance etc. ~
• The direct and reverse processes must take place infinitely slowly.
• The temperature of the system must not differ appreciably from its surroundings.
Irreversible process: Any process which is not reversible exactly is an irreversible process. All natural processes such as conduction, radiation, radioactive decay etc. are irreversible. All practical processes such as free expansion, Joule-Thomson expansion, electrical heating of a wire are also irreversible.
(a) In this case internal energy of the rod is increased from external work done by hammer which in turn increases its temperature. So, the process cannot be retraced itself.
(b) In this process energy in the form of heat is transferred to the gas in the small container by big reservoir at temperature T2.
(c) In a quasi-static isothermal expansion, the gas is ideal, this process is reversible because the cylinder is fitted with frictionless piston.
(d) As the weight is added to the cylinder arrangement in the form of external pressure hence, it cannot be reversed back itself.
Q8. An ideal gas undergoes isothermal process from some initial state i to final state f Choose the correct alternatives
![]()
Sol: (a, d)
Key concept: First Law of Thermodynamics:
It is a statement of conservation of energy in thermodynamical process.
According to it heat given to a system (∆Q) is equal to the sum of increase in its internal energy (AIT) and the work done (AW) by the system against the surroundings.
∆Q=∆U+∆W
According to the first law of thermodynamics. ∆AQ = ∆U + ∆Wbut
∆U ∝∆T
∆U=0 [As ∆T= 0]
∆Q = ∆W, i.e., heat supplied in an isothermal change is used to do work against external surrounding.
or if the work is done on the system then equal amount of heat energy will be liberated by the system
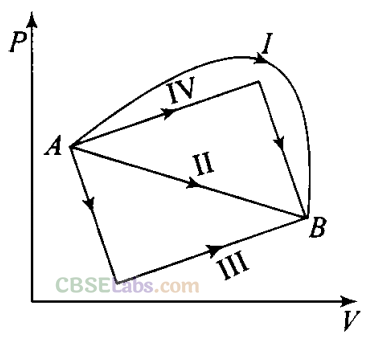
Q9. Figure shows the P-V diagram of an ideal gas undergoing a change of state from A to B. Four different parts I, II, III and IV as shown in the figure may lead to the same change of state.
(a) Change in internal energy is same in IV and III cases, but not in I and II.
(b) Change in internal energy is same in all the four cases.
(c) Work done is maximum in case I.
(d) Work done is minimum in case II.
Sol: (b, c)
Key concept: Internal energy (U): Internal energy of a system is the energy possessed by the system due to molecular motion and molecular configuration.
The energy due to molecular motion is called internal kinetic energy UK and that due to molecular configuration is called internal potential energyUp.
i.e., Total internal energy U= UK+ UP
(i) For an ideal gas, as there is no molecular attraction UP = 0
i.e., internal energy of an ideal gas is totally kinetic and is given by
U = UK = 3/2 RT
Change in internal energy does not depend on the path of the process. So it is called a point function, i.e. it depends only on the initial and final states (A and B) of the system, i.e. ∆U = Uf – Ui
Hence internal energy is same for all four paths I, II, III and IV.
The work done by an ideal gas is equal to the area bounded between P-V curve.
Work done from A to B, ∆WA→B = Area under the P-V curve which is maximum for the path I.
Q10. Consider a cycle followed by an engine (figure).
1 to 2 is isothermal
2 to 3 is adiabatic
3 to 1 is adiabatic
Such a process does not exist, because
(a) heat is completely converted to mechanical energy in such a process, which is not possible
(b) mechanical energy is completely converted to heat in this process, which is not possible
(c) curves representing two adiabatic processes don’t intersect
(d) curves representing an adiabatic process and an isothermal process don’t intersect
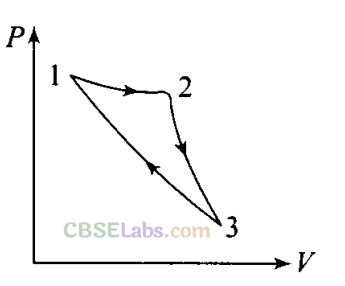
Sol. (a, c)
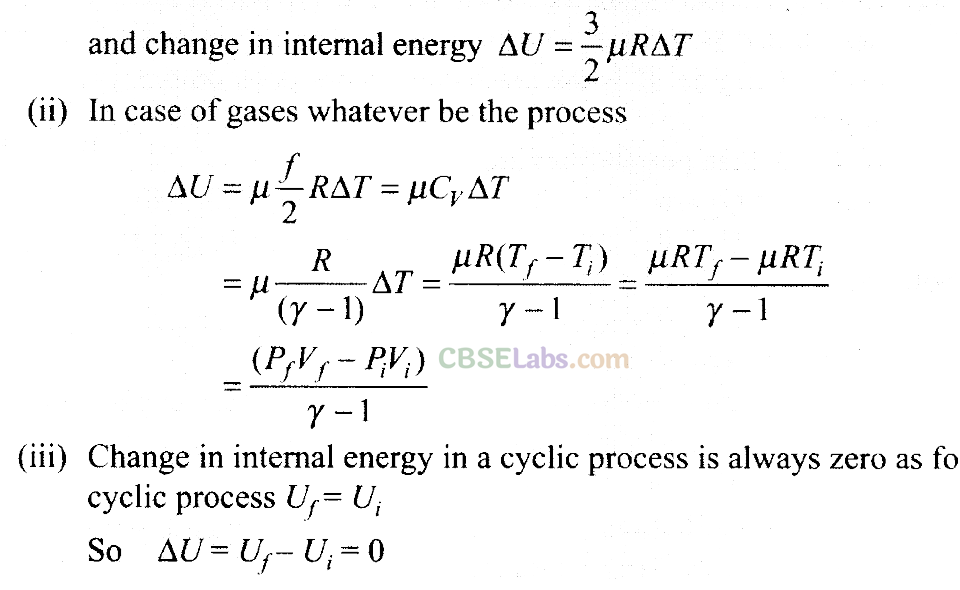
(a) The given process is a cyclic process, i.e. it returns to the original state 1. And change in internal energy in a cyclic process is always zero as for cyclic process Uf = Ui So, ∆U = Uf – Ui = 0
Hence, total heat is completely converted to mechanical energy. Such a process is not possible by second law of thermodynamics.
(c) Here, two curves are intersecting, when the gas expands adiabatically from 2 to 3. It is not possible to return to the same state without being heat supplied, hence the process 3 to 1 cannot be adiabatic. So, we conclude that such a process does not exist because curves representing two adiabatic processes do not intersect.
Q11. Consider a heat engine as shown in figure. Q1 and Q2 are heat added both to T1 and heat taken from T2 in one cycle of engine. W is the mechanical work done on the engine.If W > 0, then possibilities are:
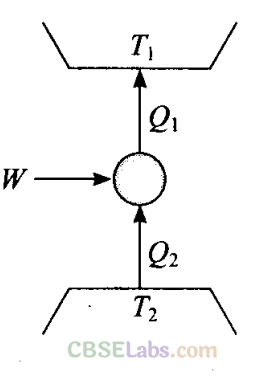

Sol: (a, c)
Key concept: Refrigerator or Heat Pump:
A refrigerator or heat pump is basically a heat engine run in reverse direction. It essentially consists of three parts:
Source: At higher temperature T1
Working substance: It is called refrigerant liquid ammonia and freon works as a working substance.
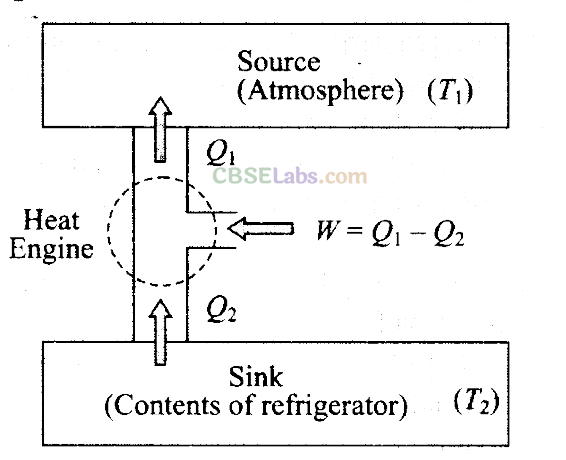
Sink: At lower temperature T2.
The working substance takes heat Q2 from a sink (contents of refrigerator) at lower temperature, has a net amount of work done W on it by an external agent (usually compressor of refrigerator) and gives out a larger amount of heat Q1, to a hot body at temperature T1 (usually atmosphere). Thus, it transfers heat from a cold body to a hot body at the expense of mechanical energy supplied to it by an external agent. The cold body is thus cooled more and more.
We know that the diagram represents the working of a refrigerator. So, we can write
Very Short Answer Type Questions
Q12. Can a system be heated and its temperature remains constant?
Sol: Yes, this is possible when the entire heat supplied to the system is utilised in expansion.
As ∆Q = ∆U + ∆W and ∆U = nCv∆T
∆Q = nCv∆T+ ∆W
If temperature remains constant, then ∆T = 0, this implies ∆Q = ∆W. This implies that heat supplied should perform work against the surroundings.
Q13. A system goes from P to Q by two different paths in the P-V diagram as shown in figure.
Heat given to the system in path 1 is 1000 J.
The work done by the system along path 1 is more than path 2 by 100 J. What is the heat exchanged by the system in path 2?
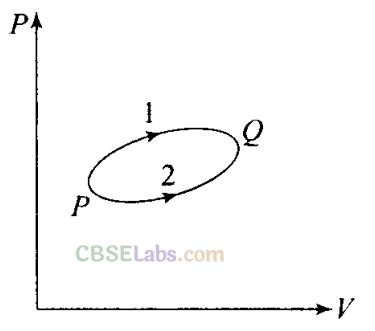
Sol: According to the first law of thermodynamics,
∆Q = AU + ∆W. Let us apply this for each path.
For path 1: Heat given Q1 = +1000 J
Let work done for path 1 = W1 .
For path 2:
Work done (W2) = (WI– 100) J
Heat given Q2 – ?
As change in internal energy between two states for different path is same.
∆ U=Qi-W1 = Q2-W2
1000– W!=Q2-(W1 – 100)
=> Q2= 1000- 100 = 900 J
Q14. If a refrigerator’s door is kept open, will the room become cool or hot? Explain.
Sol: A refrigerator is a heat engine it extracts heat from low temperature reservoir and transfer it to high temperature. If a refrigerator’s door is kept open, then room will become hot, because then refrigerator exhaust more heat into the room than earlier. In this way, temperature of the room increases and room becomes hot. No refrigerator is efficient. Thus it exhaust more heat into the room than it extract from it. Thus, a room cannot be cooled by keeping the door of a refrigerator open.
Q15. Is it possible to increase the temperature of a gas without adding heat to it? Explain.
Sol: Yes, it is possible to increase the temperature of a gas without adding heat to it, during adiabatic compression the temperature of a gas increases while no heat is given to it.
For an adiabatic compression, no heat is given or taken out in adiabatic process.
Therefore, ∆Q = 0
According to the first law of thermodynamics,
∆Q=∆U+∆W
∆U = -∆W ( ∆Q =0)
In compression work is done on the gas, i.e. work done is negative. Therefore, ∆U = Positive
Hence, internal energy of the gas increases due to which its temperature increases.
Q16 Air pressure in a car tyre increases during driving. Explain.
Sol: Volume of a car tyre is fixed. During driving, temperature of the gas increases while its volume remains constant. So, according to Charle’s law, at constant volume (V),
Pressure (P) ∝Temperature (T)
Therefore, pressure of gas increases
Short Answer Type Questions
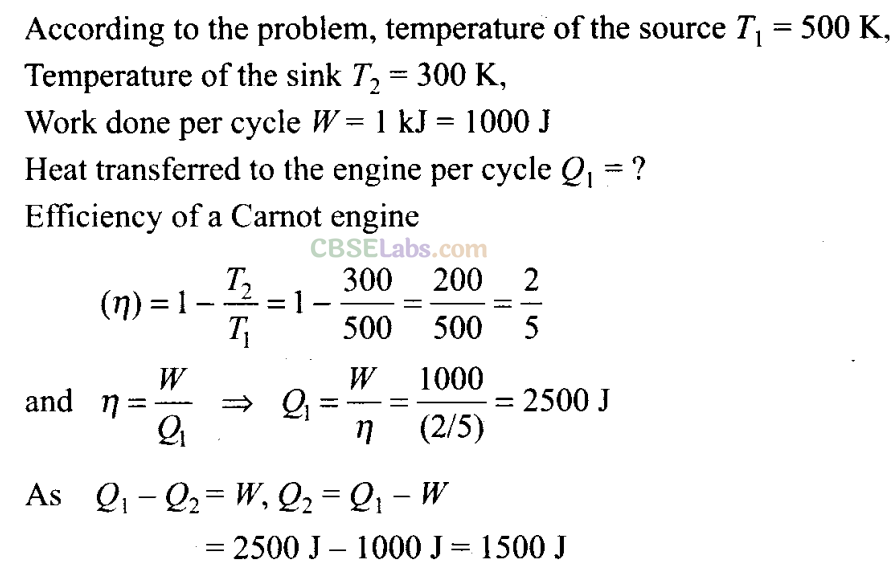
Q17. Consider a Carnot’s cycle operating between T1 = 500 K and T2 = 300 K producing 1 kJ of mechanical work per cycle. Find the heat transferred to the engine by the reservoirs.
Sol: Key concept: Carnot theorem: The efficiency of Carnot’s heat engine depends only on the temperature of source (T1) and temperature of sink(T2), and heat supplied (Q1) i.e., η= W/ Q1 = 1 – T2/ T1
(The efficiency of engine is defined as the ratio of work done to the heat supplied.)
Carnot stated that no heat engine working between two given temperatures of source and sink can be more efficient than a perfectly reversible engine (Carnot engine) working between the same two temperatures. Carnot’s reversible engine working between two given temperatures is considered to be the most efficient engine.
Q18. A person of mass 60 kg wants to lose 5 kg by going up and down a 10 m high stairs. Assume he bums twice as much fat while going up than coming down. If 1 kg of fat is burnt on expending 7000 kcal, how many times must he go up and down to reduce his weight by 5 kg?
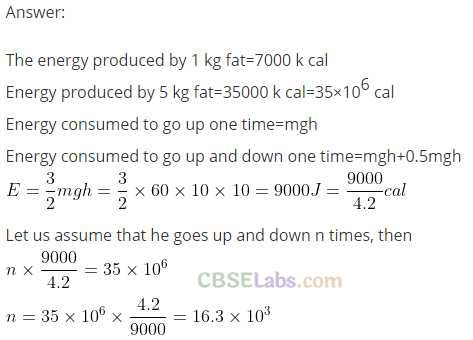
Q19. Consider a cycle tyre being filled with air by a pump. Let Vbe the volume of the tyre (fixed) and at each stroke of the pump ∆V(<< V) of air is transferred to the tube adiabatically. What is the work done when the pressure in the tube is increased from Pl to P2
Sol: Since the process is adiabatic, there is no exchange of heat in the process, Let, pressure is increased by AP and volume is increased by AV at each stroke.
For just before and after an stroke, we can write


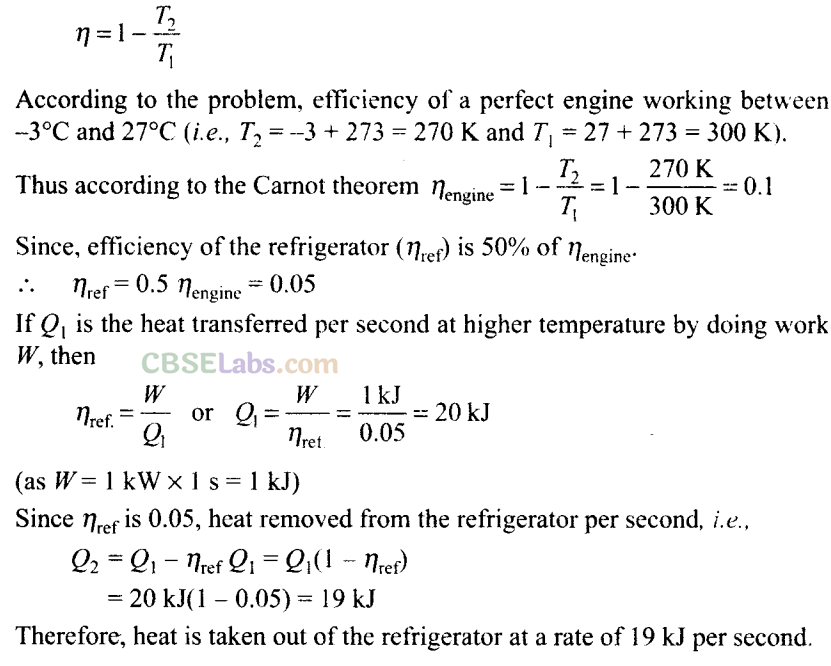
Q20. In a refrigerator one removes heat, from a lower temperature and deposits to the surroundings at a higher temperature. In this process, mechanical work has to be done, which is provided by an electric motor. If the motor is of 1 kW power and heat transferred from -3°C to 27°C, find the heat taken out of the refrigerator per second assuming its efficiency is 50% of a perfect engine.
Sol: Carnot designed a theoretical engine which is free from all the defects of a practical engine. The Carnot engine is the most efficient heat engine operating between two given temperatures. The efficiency of Carnot engine is
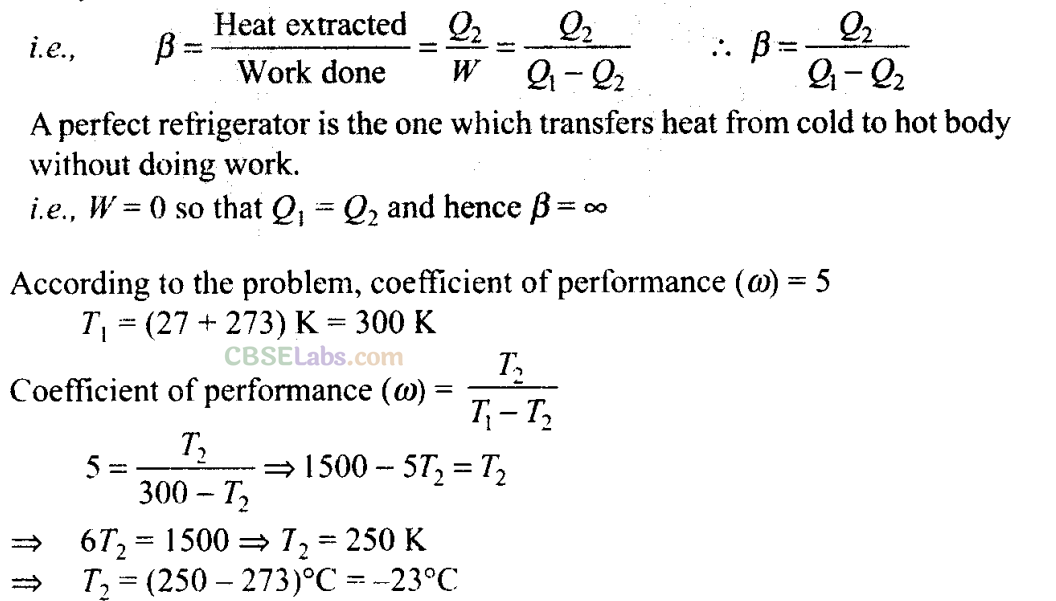
Q21. If the coefficient of performance of a refrigerator is 5 and operates at the room temperature (27°C), find the temperature inside the refrigerator.
Sol:
Key concept: The performance of a refrigerator is expressed by means of “coefficient of performance” β which is defined as the ratio of the heat extracted from the cold body to the work needed to transfer it to the hot body.
Q22. The initial state of a certain gas is (Pi,Vi Ti). It undergoes expansion till its volume becomes Vf Consider the following two cases.
a)the expansion takes place at constant temperature.
b)the expansion takes place at constant pressure.
Plot the P-V diagram for each case. In which of the two cases, is work done by the gas more?
Sol:
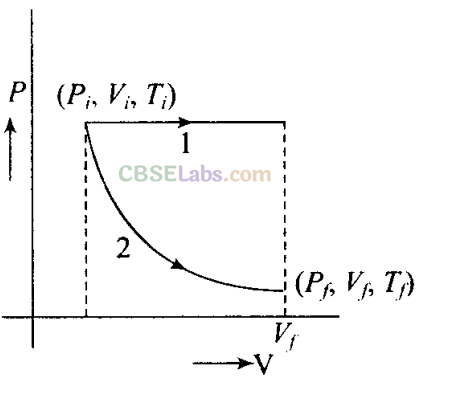
The situation is shown in the given P-V graph, where variation is shown for each process.
It is clear from the graph that Process 1 is isobaric and Process 2 is isothermal.
Since, work done is equal to the area under the P-V curve. Here, area under the P-V curve 1 is more. So, work done is more when the gas expands in isobaric process as in comparison of gas expands in isothermal.
Long Answer Type Questions
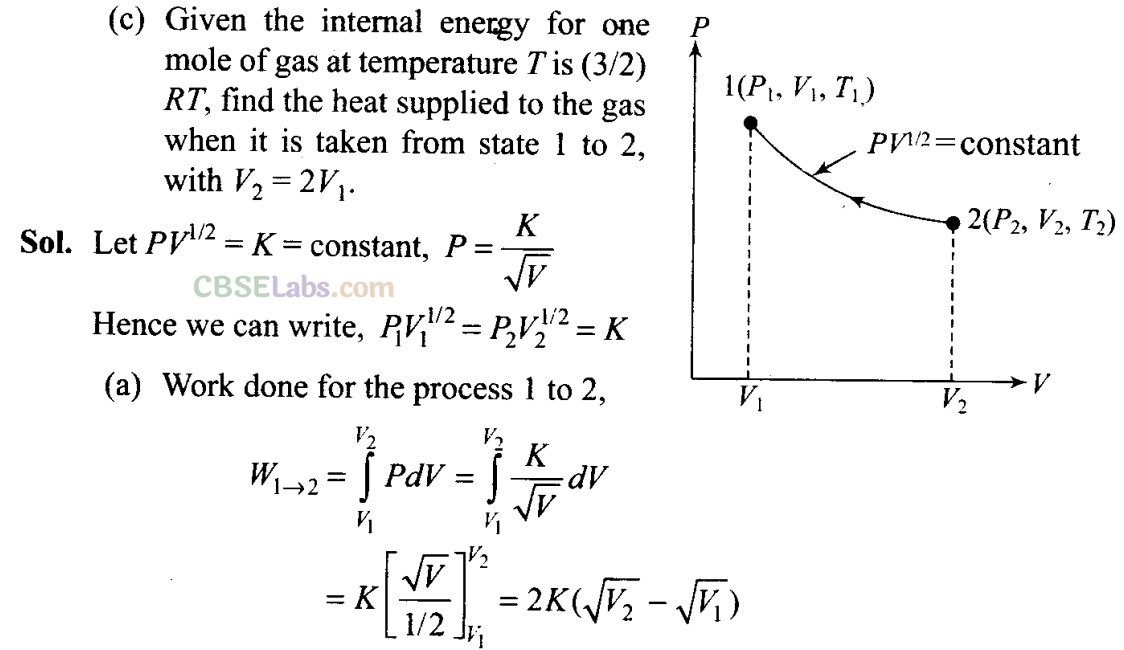
Q23. Consider a P-V diagram in which the path followed by one mole of perfect gas in a cylindrical container is shown in figure.
(a) Find the work done when the gas is taken from state 1 to state 2.
(b) What is the ratio of temperature T1/T2, if V2 = 2V1?
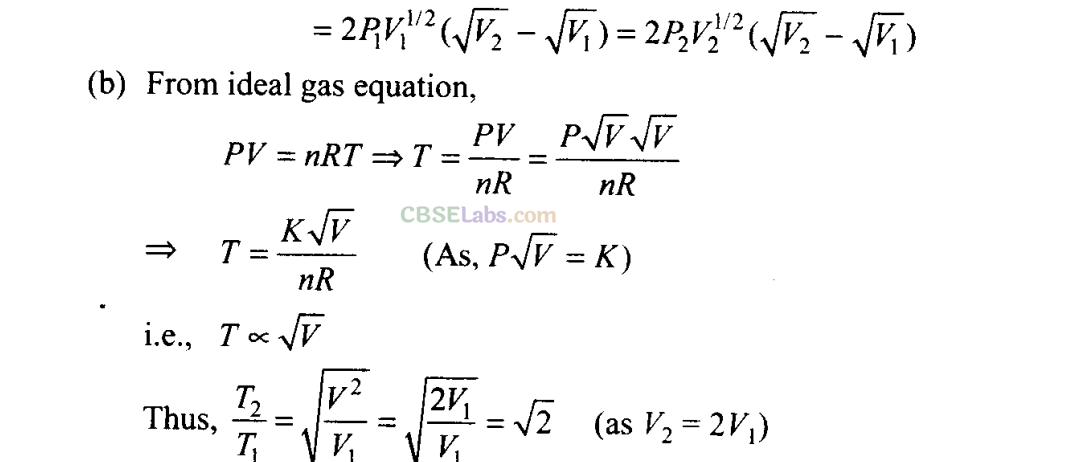
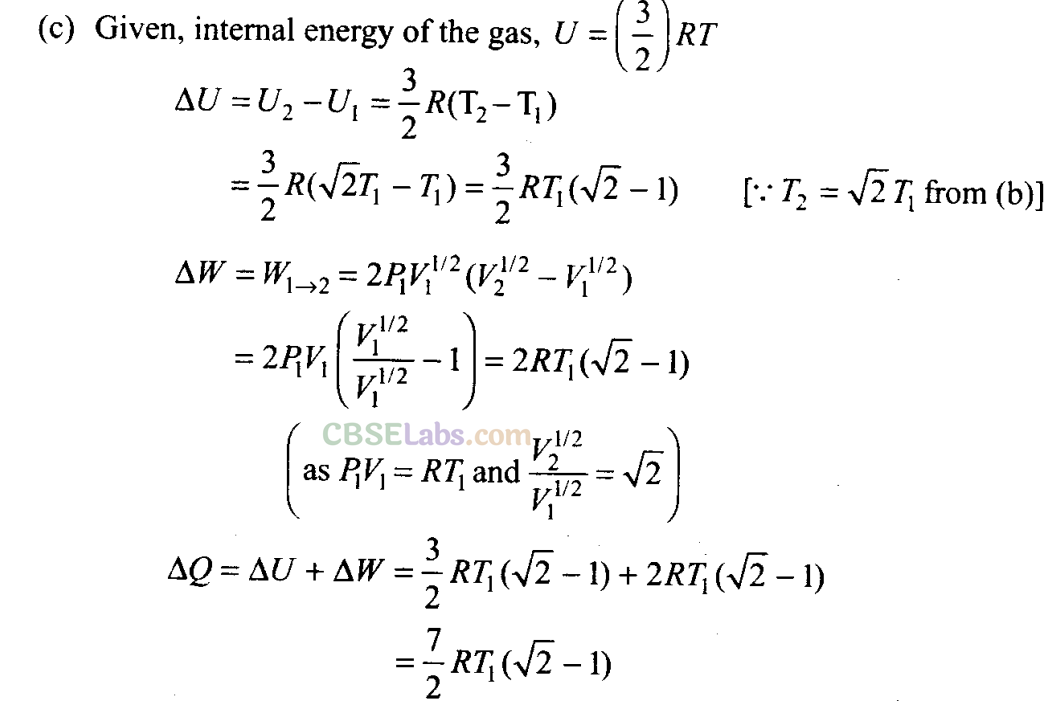
This is the amount of heat supplied.
Q24. A cycle followed by an engine (made of one mole of perfect gas in a cylinder with a piston) is shown in figure.
A to B: volume constant
B to C: adiabatic
C to D: volume constant
D to A: adiabatic
Vc =VD =2VA = 2VB
(a) In which part of the cycle heat is supplied to the engine from outside?
(b) In which part of the cycle heat is being given to the surrounding by the engine?
(c) What is the work done by the engine in one cycle? Write your answer in term of PA, PB, VA?
(d) What is the efficiency of the engine?
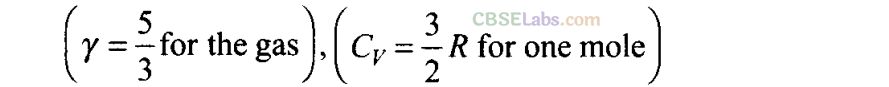
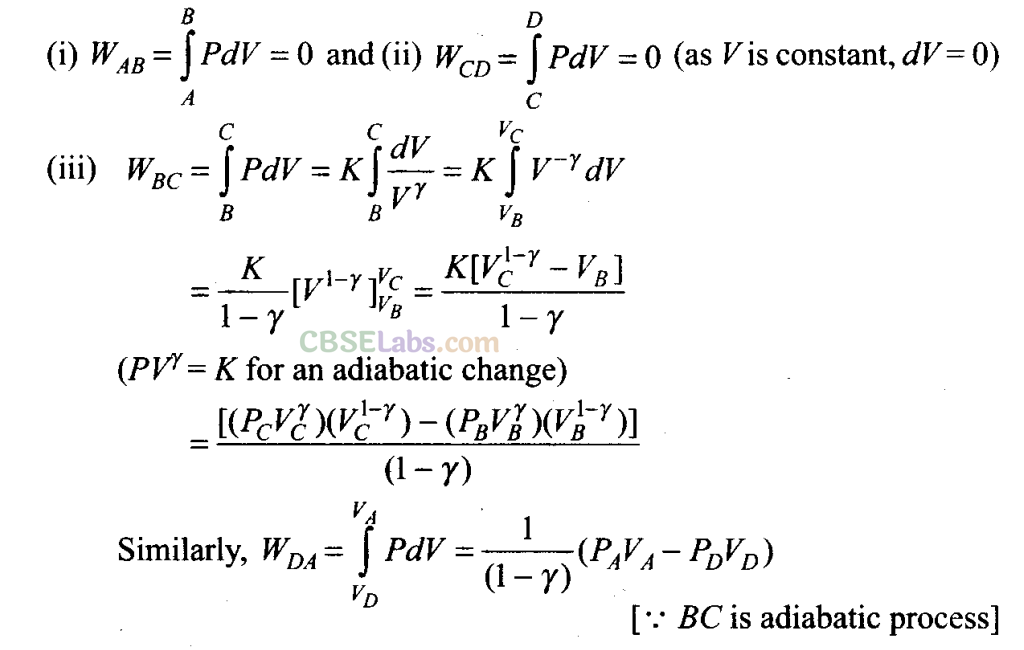
Sol: (a) For the process AB (which is isochoric process), volume is constant. So,
dV= 0 => dW= 0
dQ = dU + dW = dU
=> dQ = dU = Change in internal energy
Hence, in this process heat supplied is utilised to increase, internal energy of the system.
(b) For the process CD (which is also isochoric process), volume is constant but pressure decreases.
Hence, temperature also decreases (because Pα T) so heat is given to the surroundings.
(c) To calculate work done by the engine in one cycle, we calculate work done in each part separately.
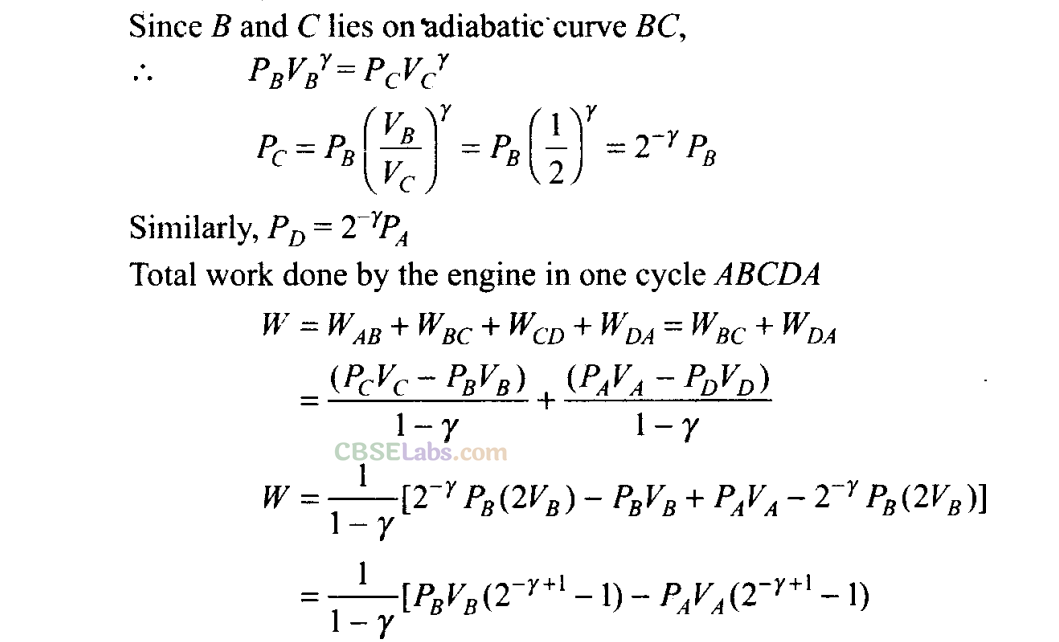
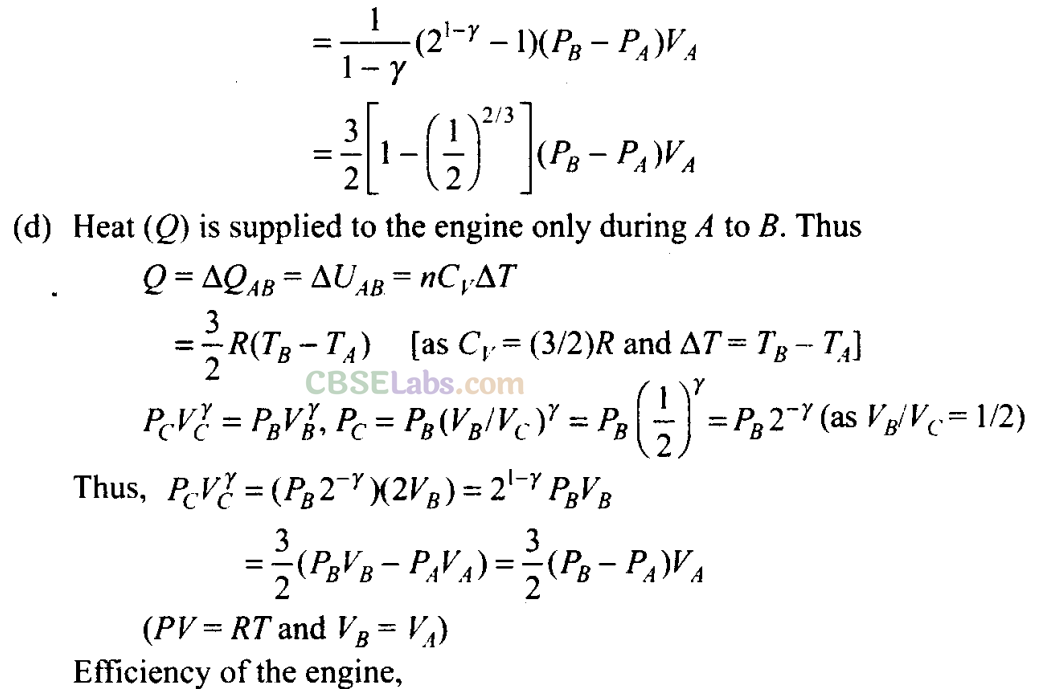
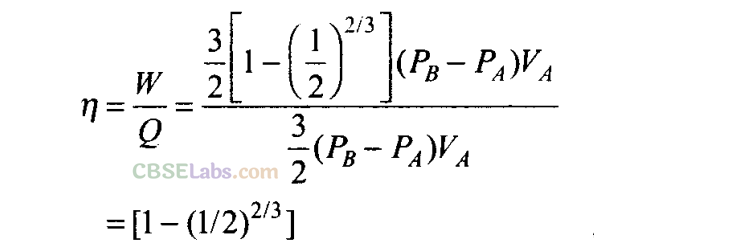
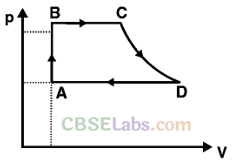
Q25. A cycle followed by an engine (made of one mole of an ideal gas in a cylinder with a piston) is shown in figure. Find heat exchanged by the engine, with the surroundings for each section of the cycle. [Cv = (3/2)/?]
(a) AB: constant volume
(b) BC: constant pressure
(c) CD: adiabatic
(d) DA : constant pressure
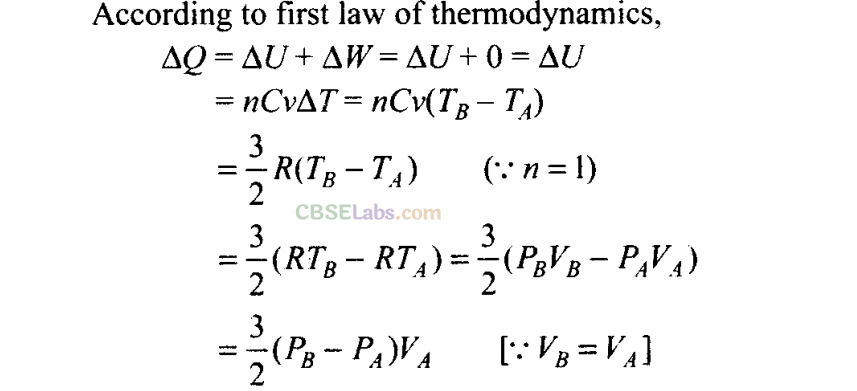
Sol: (a)
By using first law of thermodynamics, we can find amount of heat associated with each process
For process AB
Volume is constant, hence work done dW = 0
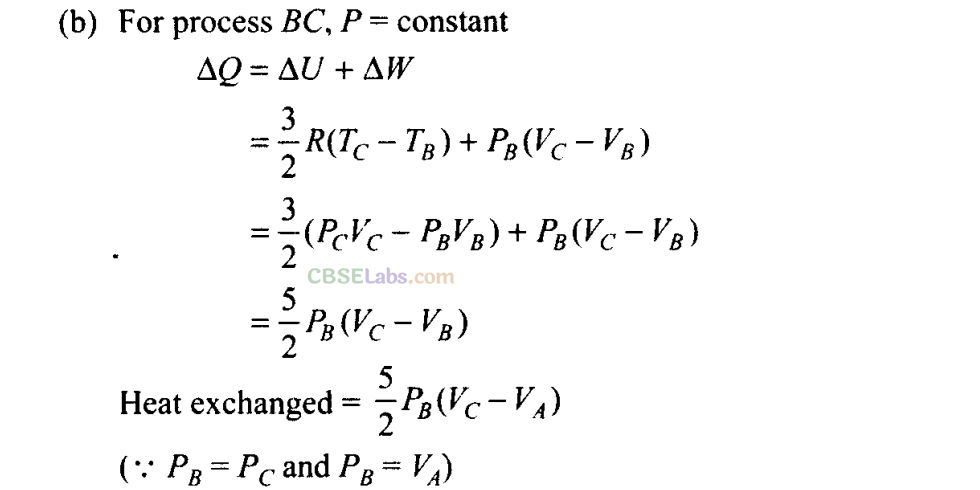
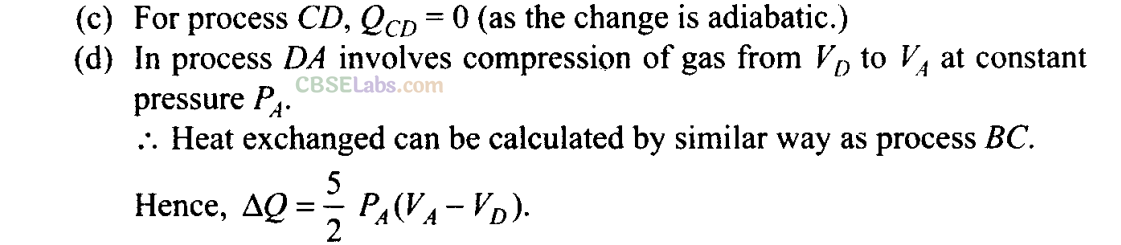
NCERT Exemplar Class 11 Physics Solutions
- Chapter 1 Units and Measurements
- Chapter 2 Motion in a Straight Line
- Chapter 5 Work, Energy and Power
- Chapter 6 System of Particles and Rotational Motion
- Chapter 8 Mechanical Properties of Solids
- Chapter 9 Mechanical Properties of Fluids
- Chapter 10 Thermal Properties of Matter
- Chapter 11 Thermodynamics
- Chapter 13 Oscillations
- Chapter 14 Waves
NCERT Exemplar ProblemsMathsPhysicsChemistryBiology
We hope the NCERT Exemplar Class 11 Physics Chapter 11 Thermodynamics help you. If you have any query regarding NCERT Exemplar Class 11 Physics Chapter 11 Thermodynamics, drop a comment below and we will get back to you at the earliest.