Important Questions for Class 12 Chemistry Chapter 5 Surface Chemistry Class 12 Important Questions
Surface Chemistry Class 12 Important Questions Very Short Answer Type
Question 1.
Define the term ‘Tyndall effect’. (Delhi 2009)
Answer:
Tyndall effect : When a beam of light is passed through a colloidal solution and viewed perpendicular to the path of the incident light, the path of light becomes visible as a bright streak. The illuminated path is called Tyndall cone and the phenomenon is called Tyndall effect.
Question 2.
What is the ‘coagulation’ process? (All India 2009)
Answer:
The process of settling of colloidal particles is called coagulation or precipitation of the solution.
Question 3.
What is an emulsion? (Delhi 2010)
Answer:
Emulsion is liquid-liquid colloidal system.
Question 4.
Give an example of ‘shape-selective catalyst’. (Delhi 2010)
Answer:
The catalyst reaction in which small sized molecules are absorbed in the pores and cavities of selective adsorbents like zeolites is known as shape-selective catalysis.
Question 5.
Define ‘electrophoresis’. (Delhi 2011)
Answer:
Electrophoresis : When electric current is passed through a colloidal solution, the positively charged particles move towards cathode while negatively charged particles move towards anode where they lose their charge and get coagulated. The phenomenon is known as Electrophoresis.
Question 6.
What is meant by ‘shape-selective catalysis’ of reactions? (All India 2011)
Answer:
The catalyst reaction in which small sized molecules are absorbed in the pores and cavities of selective adsorbents like zeolites is known as shape-selective catalysis.
Question 7.
What are lyophobic colloids? Give one example for them. (All India 2011)
Answer:
Lyophobic sols : Substances like metals, their sulphides, etc., when simply mixed with the dispersion medium do not form the colloidal sol. Their colloidal sols can only be prepared by specific methods. They are not much hydrated and are irreversible in nature. They are also called extrinsic colloids.
Example : AS2S3 sol.
Question 8.
Define ‘peptization’. (All India 2012)
Answer:
Peptization may be defined as the process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte.
Question 9.
What is meant by ‘shape selective catalysis’? (All India 2012)
Answer:
The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis.
Question 10.
Out of NH3 and CO2 which gas will be adsorbed more readily on the surface of activated charcoal and why? (Comptt. Delhi 2012)
Answer:
NH3 gas will be adsorbed more readily on activated charcoal. It has higher critical temperature than CO2 and is an easily liquifiable gas. Its van der Waals forces are stronger.
Question 11.
How can a colloidal solution and true solution of the same colour be distinguished from each other? (Comptt. Delhi 2012)
Answer:
A colloidal solution scatters a beam of light but a true solution does not.
Question 12.
How is a sol different from an emulsion ? (Comptt. All India 2012)
Answer:
A collidal sol contains solid as the dispersed phase and liquid as the dispersion medium e.g. paint, gold sol etc.
Emulsion : A colloidal dispersion in which the dispersed- phase and the dispersion medium are immiscible liquids, is known as emulsion e.g. milk, butter etc.
Question 13.
Write two applications of adsorption. (Comptt. All India 2012)
Answer:
Applications of adsorption :
- In decolorisation of sugar.
- In gas masks, charcoal is used which adsorbs poisonous gases in mines.
Question 14.
Why do true solutions not show Tyndall effect? (Comptt. All India 2012)
Answer: In true solution, the diameter of the dispersed particles is much smaller than the wavelength of the light used, hence there is no scattering of light.
Question 15.
Of physisorption or chemisorption, which has a higher enthalpy of adsorption? (All India 2013)
Answer:
Chemisorption has higher enthalpy of adsorption than physisorption due to chemical bond formation.
Question 16.
What is especially observed when a beam of light is passed through a colloidal solution? (All India 2013)
Answer:
Tyndall effect is observed when a beam of light is passed through a colloidal solution.
Question 17.
To which colloidal system does milk belong? (Comptt. All India 2013)
Answer:
Milk belongs to emulsion.
Question 18.
What is electrophoresis due to? (Comptt. All India 2013)
Answer:
Electrophoresis is due to electrical charge on the colloidal particles.
Question 19.
What is meant by the term peptization? (Comptt. All India 2013)
Answer:
Peptization may be defined as the process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte.
Question 20.
Give one example each of ‘oil in water’ and ‘water in oil’ emulsion. (Delhi 2014)
Answer:
Oil in water → Milk, vanishing cream
Water in oil → Butter, cold creams.
Question 21.
Give one example each of sol and gel. (Delhi 2014)
Answer:
Sol → Smoke, dust
Gel → Cheese
Question 22.
Give one example each of lyophobic sol and lyophilic sol. (Delhi 2014)
Answer:
Lyophobic sol — Metal sulphides
Lyophilic sol — Starch
Question 23.
What is the effect of temperature on chemisorption? (All India 2014)
Answer:
Chemisorption increases with increase of temperature.
Question 24.
Why is adsorption always exothermic? (All India 2014)
Answer:
Adsorption is accompanied by decrease of randomness. For the process to be spontaneous,
ΔG must be negative.
Hence, according to equation ΔG = ΔH – TΔS, ΔG can be -ve only if ΔH is negative.
Question 25.
What are the dispersed phase and dispersion medium in milk? (All India 2014)
Answer:
Milk : Dispersed phase → Fat (Liquid);
Dispersion medium → Liquid
Question 26.
What is the difference between lyophobic sol and lyophilic sol? (Comptt. Delhi 2014)
Answer:
Lyophobic sols: Substances like metals, their sulphides, etc., when simply mixed with the dispersion medium do not form the colloidal sol. Their colloidal sols can only be prepared by specific methods. They are not much hydrated and are irreversible in nature. They are also called extrinsic colloids.
Example : AS2S3 sol.
Lyophilic sols: Liquid loving colloids in which there is affinity between disperse phase and dispersion medium.
Example : Starch sol, Gum sol, Gelatin sol
Question 27.
What is a ‘shape-selective catalyst’? (Comptt. Delhi 2014)
Answer:
The catalyst reaction in which small sized molecules are absorbed in the pores and cavities of selective adsorbents like zeolites is known as shape-selective catalysis.
Question 28.
What are emulsions? Name an emulsion in which water is a dispersed phase. (Comptt. All India 2014)
Answer:
Emulsions : An emulsion is a colloidal dispersion in which both the dispersed phase and dispersion medium are liquids.
Water in oil → Butter, cold creams.
Question 29.
Define dialysis. (Comptt. All India 2014)
Answer:
Dialysis : The process of separating the particles of colloids from those of crystalloids by diffusion of the mixture through a parchment or an animal membrane is known as dialysis.
Question 30.
A delta is formed at the melting point of sea water and river water. Why? (All India 2015)
Answer:
Delta is formed at the meeting point of sea water and river water due to coagulation of colloidal clay particles.
Question 31.
In reference to surface chemistry, define dialysis. (Comptt. Delhi 2015)
Answer:
Dialysis : The process of removing the dissolved substances from a colloidal solution by means of diffusion through a suitable membrane is called dialysis.
Question 32.
What are emulsions? Give an example. (Comptt. All India 2015)
Answer:
Emulsions : An emulsion is a colloidal dispersion in which both the dispersed phase and dispersion medium are liquids. For example, milk, cream.
Question 33.
Why is adsorption always exothermic? (Comptt. Delhi 2016)
Answer:
Due to the force of attraction/bond formation between adsorbate and adsorbent.
Question 34.
Why is Tyndall effect shown by colloidal solutions? (Comptt. All India 2016)
Answer:
It is so due to large size of colloidal particles. In colloidal solutions particle size of dispersed phase is comparable to the wavelength of light used.
Question 35.
What are associated colloids? Given an example. (Comptt. All India 2016)
Answer:
Colloids which act as electrolyte at low concentration and show colloidal behaviour at high concentration are called Associated colloids. Example : Soap solution, Detergents.
Question 36.
Write one similarity between physisorption and chemisorption. (Delhi 2017)
Answer:
Both physisorption and chemisorption increase with increase in pressure. Both increase with increase in surface area.
Question 37.
What type of colloid is formed when a liquid is dispersed in a solid? Give an example. (All India) 2017
Answer:
Gel is formed when a liquid is dispersed in a solid, e.g., Cheese, butter, etc.
Question 38.
What type of colloid is formed when a solid is dispersed in a liquid? Give an example. (All India 2017)
Answer:
Sol is formed when a solid is dispersed in a liquid, e.g., paints.
Question 39.
What type of colloid is formed when a gas is dispersed in a liquid? Give an example. (All India 2017)
Answer:
Foam is formed when a gas is dispersed in a liquid, e.g., Froth.
Question 40.
Which of the following is most effective in coagulating negatively charged hydrated ferric oxide sol? (Comptt. Delhi 2017)
(i) NaN03 (ii) MgSO4 (iii) AlCl3
Answer:
AlCl3 (Aluminium chloride) is most effective in coagulating negatively charged hydrated ferric oxide sol.
Question 41.
Which of the following is most effective in coagulating positively charged hydrated ferric oxide sol? (Comptt. Delhi) 2017
(i) NaNO3 (ii) Na2SO4 (Hi) (NH4)3PO4
Answer:
Ammonium phosphate (NH4)3PO4 is most effective in coagulating positively charged hydrated ferric oxide sol.
Question 42.
Which of the following is most effective in coagulating positively charged methylene blue sol? (Comptt. Delhi 2017)
(i) Na3PO4 (ii) K4[Fe(CN)6] (iii) Na2SO4
Answer:
Potassium ferrocyanide K4[Fe(CN)6]
Question 43.
What are emulsions? Give an example. (Comptt. All India 2017)
Answer:
An emulsion is a colloidal dispersion in which both the dispersed phase and dispersion medium are liquids. For example, milk, cream.
Question 44.
Write the dispersion medium and dispersed phase in milk. (Comptt. All India 2017)
Answer:
Liquid fat is the dispersed phase and water is the dispersion medium.
Question 45.
Write the dispersed phase and dispersion medium in butter. (Comptt. All India 2017)
Answer:
Water is the dispersed phase and oil is the dispersion medium in butter.
Surface Chemistry Class 12 Important Questions Short Answer Type [SA – I]
Question 46.
Describe the following :
(i) Tyndall effect
(ii) Shape-selective catalysis (All India 2010)
Answer:
(i) Tyndall effect : When a beam of light is passed through a colloidal solution and viewed perpendicular to the path of the incident light, the path of light becomes visible as a bright streak. The illuminated path is called Tyndall cone and the phenomenon is called Tyndall effect.
(ii) Shape-selective catalysis : The catalyst reaction in which small sized molecules are absorbed in the pores and cavities of selective adsorbents like zeolites is known as shape-selective catalysis.
Question 47.
What is meant by coagulation of a colloidal solution? Name any method by which coagulation of lyophobic sols can be carried out. (All India 2010)
Answer:
Coagulation : The process of settling of colloidal particles is called coagulation or precipitation of the solution.
Name of method : The method which can be used for coagulation of lyophobic solutions is electrophoresis.
Question 48.
Define the following :
(i) Peptization (ii) Reversible sols (All India 2010)
Answer:
(i) Peptization : It is a process of converting a fresh precipitate into colloidal particles by shaking it with the dispersion medium in the presence of a small amount of a suitable electrolyte.
(ii) Reversible sols : The lyophilic solutions in which if the dispersed phase is separated from the dispersion medium, the sol can be made again by simply remixing with the dispersion medium. This shaking is called as Reversible sol.
Question 49.
Name the two groups into which phenomenon of catalysis can be divided. Give an example of each group with the chemical equation involved. (Delhi 2012)
Answer:
Homogeneous catalysis : When the reactants and the catalyst are in the same phase, the process is called homogeneous catalysis.
Example : 2SO2 (g) + O2 (g) \(\underrightarrow { NO\left( g \right) } \) 2SO3 (g)
Heterogeneous catalysis : When the reactants and the catalyst are in different phases, the process is called heterogeneous catalysis.
Example : 2SO2 (g) \(\underrightarrow { Pt\left( s \right) } \) 2SO3 (g)
Question 50.
What is meant by coagulation of a colloidal solution? Describe briefly any three methods by which coagulation of lyophobic sols can be carried out. (Delhi 2012)
Answer:
The process of setting of colloidal particles is called coagulation or precipitation of the sol. Methods of coagulation :
- By electrophoresis : The colloidal particles move towards oppositely charged electrodes.
- By mixing two oppositely charged sols.
- By addition of electrolytes : When excess of an electrolyte is added, the colloidal particles are precipitated. ‘
Question 51.
Describe a conspicuous change observed when
(i) a solution of NaCl is added to a sol. of hydrated ferric oxide.
(ii) a beam of light is passed through a solution of NaCl and then through a sol. (Delhi 2012)
Answer:
(i) Coagulation or precipitation of sol. takes place.
(ii) Scattering of light is observed when light is passed through a sol. whereas no scattering of light is observed in a solution of NaCl/ , Tyndall effect in the sol.
Question 52.
Explain the following terms giving one example for each :
(i) Miscelles (ii) Aerosol (Delhi 2012)
Answer:
(i) Miscelles : They are associated colloids showing colloidal behaviour at high concentration and strong electrolytes at low concentration.
Example : soap, detergent.
(ii) Aerosol : Aerosol is a colloidal solution of solid or liquid (dispersed phase) in gas (dispersion medium).
Example : smoke, dust, fog, mist, cloud.
Question 53.
Write the dispersed phase and dispersion medium of the following colloidal systems:
(i) Smoke
(ii) Milk (Delhi 2013)
Answer:
(i) Smoke: Dispersed Phase → Solid;
Dispersed medium → Gas;
(ii) Milk: Dispersed Phase → Fat (Liquid);
Dispersed medium Liquid
Question 54.
What are lyophilic and lyophobic colloids? Which of these sols can be easily coagulated on the addition of small amounts of electrolytes? (Delhi 2013)
Answer:
Lyophobic sols : Substances like metals, their sulphides, etc., when simply mixed with the dispersion medium do not form the colloidal sol. Their colloidal sols can only be prepared by specific methods. They are not much hydrated and are irreversible in nature. They are also called extrinsic colloids.
Example : AS2S3 sol.
Lyophilic sols : Liquid loving colloids in which there is affinity between disperse phase and dispersion medium.
Example : Starch sol, Gum sol, Gelatin sol
Question 55.
What is the difference between oil/water (O/W) type and water/oil (W/O) type emulsions? Give an example of each type. (Delhi 2013)
Answer:
Difference between two types of emulsions are : Emulsions of oil in water in which oil is the dispersed phase and water is the dispersion medium.
Example : Milk is an emulsion of liquid fat dispersed in water.
Emulsions of water in oil in which water is the dispersed phase and oil is the dispersion medium. e.g. Cod liver oil is an emulsion of oil i.e. water is the dispersed phase and oil is the dispersion medium.
Two applications of emulsion are :
- The digestion of fats in the intestines takes place by the process of emulsification.
- Several oily drugs are prepared in the form of emulsion.
Question 56.
What is the difference between multi-molecular and macromolecular colloids? Give one example of each. (Delhi 2013)
Answer:
Multi-molecular colloid is aggregation of large number of atoms or smaller molecules of a substance having size in the colloidal range. Whereas macromolecular colloid is the solution containing macromolecules in the colloidal range.
Example: Multimolecular colloids: Gold sol,
Sulphur sol
Macromolecular colloids: Proteins,
Cellulose (any one)
Question 57.
Explain the following :
(a) Same substance can act both as colloids and crystalloids.
(b) Artificial rain is caused by spraying salt over clouds. (Comptt. Delhi 2013)
Answer:
(a) Sodium chloride behaves as a crystalloid when dissolved in water but behaves as a colloid when dissolved in benzene.
(b) Artificial rain is caused by spraying common salt over the clouds, as it is an electrolyte and brings about coagulation of water particles.
Question 58.
How are the following colloidal solutions prepared?
(a) Sulphur in water
(b) Gold in water (Comptt. Delhi 2013)
Answer:
(a) Sulphur in water : An alcoholic solution (true solution) of sulphur in excess of water is added, then colloidal solution is obtained. Or, Oxidation of H2S
HNO3 + H2 S → H2O + NO2 + S
(b) Gold in water : Gold solution can be prepared by reduction of AuCl3 solution with formaldehyde.
2AuCl3 + 3HCHO + 3H2O → 2Au Gold Sol. + 3HCOOH + 6HCl
Question 59.
What is an adsorption isotherm? Describe Freundlich adsorption isotherm.(Comptt. Delhi 2013)
Answer:
Adsorption isotherm : A graph between the amount of the gas adsorbed per gram of the adsorbent (x/m) and the equilibrium pressure of the adsorbate at constant temperature is called the adsorption isotherm.
The extent of adsorption is usually expressed as x/m,
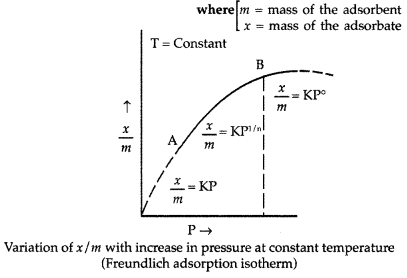
A relationship between the amount adsorbed (x/m) and the equilibrium pressure (P) can be obtained as follows :
At low values of P, the graph is nearly straight and sloping.
\(\frac{x}{m}\) ∝ P or \(\frac{x}{m}\) = constant × P1
At high pressure, x/m becomes independent of the values of P. In this range of pressure,
\(\frac{x}{m}\) ∝ P° or \(\frac{x}{m}\) = constant × P°
In the intermediate range of pressure, x/m will depend on P raised to powers between 1 and 0 i.e. fraction. For a small range of pressure values, we can write :
\(\frac{x}{m}\) ∝ P1/n or \(\frac{x}{m}\) = KP1/n
Where n = Positive integer
K = constant depends upon the nature of adsorbate and adsorbent at a particular temperature.
The factor 1 / n has values between 0 and 1.
This relationship was given by Freundlich and is known as Freundlich adsorption isotherm.
Question 60.
What are emulsions? Describe different types of emulsions giving one example of each type. (Comptt. Delhi 2013)
Answer:
Emulsion : The colloidal solution in which both dispersed phase and medium are in liquid state is called emulsion. This is liquid-liquid colloidal system.
Different types of emulsions are :
Emulsions of oil in water in which oil is the dispersed phase and water is the dispersion medium.
Example : Milk is an emulsion of liquid fat dispersed in water.
Emulsions of water in oil in which water is the dispersed phase and oil is the dispersion medium. e.g. Cod liver oil is an emulsion of oil i.e. water is the dispersed phase and oil is the dispersion medium.
Two applications of emulsion are :
- The digestion of fats in the intestines takes place by the process of emulsification.
- Several oily drugs are prepared in the form of emulsion.
Surface Chemistry Class 12 Important Questions Short Answer Type – II [SA – II]
Question 61.
How are the following colloids different from each other in respect of dispersion medium and dispersed phase? Give one example of each type.
(i) An aerosol
(ii) A hydrosol
(iii) An emulsion (Delhi 2009)
Answer:
(i) An aerosol: It is a colloidal solution in which dispersed phase is a solid and dispersion medium is a gas.
Example : Smoke, haze.
(ii) A hydrosol : The colloidal solution where water is used as the dispersion medium is called hydrosol or aquasol.
Example : Starch sol.
(iii) An emulsion : The colloidal solution in which both dispersed phase and medium are in liquid state.
Example : Milk, cream.
Question 62.
What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids different from these two types of colloids? (Delhi 2009)
Answer:
| Multimolecular colloids | Macromolecular colloids | Associated colloids |
| 1. They are formed by the aggregation of a large number of atoms or molecules which generally have diameter less than 1 mm, e.g. sols of gold, sulphur etc. | They are molecules of large size e.g. polymers like rubber, nylon, starch, proteins etc. | They are formed by aggregation of a large number of ions in concentrated solution e.g. soap sol. |
| 2. Their molecular masses are not very high. | They have high molecular masses. | Their molecular masses are generally high. |
| 3. Their atoms or molecules are held together by weak Van der Waals forces. | Due to long chain, the Van der Waals forces holding them are comparatively stronger. | Higher is the concentration, greater are the Van der Waals forces. |
Question 63.
What happens in the following activities and why?
(i) An electrolyte is added to a hydrated ferric oxide sol in water.
(ii) A beam of light is passed through a colloidal solution.
(iii) An electric current is passed through a colloidal solution. (All India 2009)
Answer:
(i) When NaCl is added to hydrated ferric oxide solution, then coagulation will take place. A negatively charged solution is obtained with absorption of OH– ion.
(ii) When electric current is passed through a colloidal solution, the positively charged particles move towards cathode while negatively charged particles move towards anode where they lose their charge and get coagulated. The phenomenon is known as Electrophoresis.
(iii) When a beam of strong light is passed through a colloidal solution, scattering of light occurs by colloidal particles and the path of light becomes visible and the phenomenon is known as Tyndall effect.
Question 64.
What is the difference between multimolecular and macromolecular colloids? Give one example of »gach type. How are associated colloids different from these two types of colloids? (Delhi 2010)
Answer:
| Multimolecular colloids | Macromolecular colloids | Associated colloids |
| 1. They are formed by the aggregation of a large number of atoms or molecules which generally have diameter less than 1 mm, e.g. sols of gold, sulphur etc. | They are molecules of large size e.g. polymers like rubber, nylon, starch, proteins etc. | They are formed by aggregation of a large number of ions in concentrated solution e.g. soap sol. |
| 2. Their molecular masses are not very high. | They have high molecular masses. | Their molecular masses are generally high. |
| 3. Their atoms or molecules are held together by weak Van der Waals forces. | Due to long chain, the Van der Waals forces holding them are comparatively stronger. | Higher is the concentration, greater are the Van der Waals forces. |
Question 65.
How are the following colloids different from each other in respect of their dispersion medium and dispersed phase? Give one example of each.
(i) Aerosol
(ii) Emulsion
(iii) Hydrosol (Delhi 2010)
Answer:
(i) An aerosol: It is a colloidal solution in which dispersed phase is a solid and dispersion medium is a gas.
Example : Smoke, haze.
(ii) A hydrosol : The colloidal solution where water is used as the dispersion medium is called hydrosol or aquasol.
Example : Starch sol.
(iii) An emulsion : The colloidal solution in which both dispersed phase and medium are in liquid state.
Example : Milk, cream.
Question 66.
Explain how the phenomenon of adsorption finds application in each of the following processes :
(i) Production of vacuum
(ii) Heterogeneous catalysis
(iii) Froth Floatation process (Delhi 2011)
Answer:
(i) Production of vacuum : In Dewar flasks, activated charcoal is placed between the walls of the flask so that any gas which enters into annular space either due to glass imperfection or diffusion through glass is adsorbed and create a vacuum.
(ii) Heterogeneous catalysis : If the catalyst is present in a different phase than that of the reactants, it is called a heterogeneous catalyst and this type of catalysis is called Heterogeneous catalysis.
Example : Manufacture of NH3 from N2 and H2 by Haber’s process using iron as catalyst
N2 (g) + 3H2 (g) \(\underrightarrow { Fe\left( s \right) } \) 2NH3 (g)
Reactants are gaseous whereas catalyst is solid.
(iii) Froth Floatation process : When KCl is added to hydrated ferric oxide sol, then a negatively charged sol is obtained with absorption of OH– ion.
Question 67.
Define each of the following terms :
(i) Micelles
(ii) Peptization
(iii) Desorption (Delhi 2011)
Answer:
(i) Micelles : The substances, which when dissolved in a medium at low concentrations behave as normal, strong electrolytes but at higher concentration exhibit colloidal state properties due to the formation of aggregated particles and such aggregated particles thus formed are called micelles.
(ii) Peptization : It is a process of converting a fresh precipitate into colloidal particles by shaking it with the dispersion medium in the presence of a small amount of a suitable electrolyte.
(iii) Desorption : The removal of adsorbed substance from the surface of a solid or liquid by heating or by reducing pressure is called desorption.
Question 68.
Classify colloids where the dispersion medium is water. State their characteristics and write an example of each of these classes. (All India 2011)
Answer:
Colloids are classified into two types in the cases where dispersion medium is water. They are:
(i) Hydrophilic or Lyophilic colloids : Those substances, which when mixed with the dispersion medium, form directly the colloidal solution and are termed as hydrophilic colloids. They are reversible solutions, quite stable and cannot be easily precipitated.
Example : gum, gelatine, starch, rubber etc.
(ii) Hydrophobic or Lyophobic colloids : Those substances which do not form colloidal solution when simply mixed with the dispersion medium, are called hydrophobic colloids. They are irreversible solutions, unstable and can be easily precipitated. Example : Metals and their sulphides
Question 69.
Explain what is observed when
(i) an electric current is passed through a sol
(ii) a beam of light is passed through a sol
(iii) an electrolyte (say NaCl) is added to ferric hydroxide sol (All India 2011)
Answer:
(i) When NaCl is added to hydrated ferric oxide solution, then coagulation will take place. A negatively charged solution is obtained with absorption of OH– ion.
(ii) When electric current is passed through a colloidal solution, the positively charged particles move towards cathode while negatively charged particles move towards anode where they lose their charge and get coagulated. The phenomenon is known as Electrophoresis.
(iii) When a beam of strong light is passed through a colloidal solution, scattering of light occurs by colloidal particles and the path of light becomes visible and the phenomenon is known as Tyndall effect.
Question 70.
Explain the following terms giving a suitable example for each :
(i) Aerosol
(ii) Emulsion
(iii) Micelle (All India 2012)
Answer:
(i) Aerosol : An aerosol is a colloid in which dispersed phase is a solid and dispersion medium is a gas.
Example : Smoke, dust
(ii) Emulsion : It is a colloidal solution of two immiscible liquids of which one is the dispersion medium while the other is the dispersed phase (liquid-liquid).
Example : Milk, cream
(iii) Micelles: They are associated colloids showing colloidal behaviour at high concentration and strong electrolytes at low concentration. Example : Soap, detergent.
Question 71.
Write three distinct features of chemisorptions which are not found in physisorptions. (All India 2012)
Answer:
Three distinct features of chemisorptions :
- It is caused by chemical bond formation.
- It is highly specific in nature.
- It is irreversible.
Question 72.
(a) Adsorption of a gas on surface of solid is generally accompanied by a decrease in entropy, still it is a spontaneous process. Explain.
(b) Some substances can act both as colloids ’ and crystalloids. Explain.
(c) What will be the charge on Agl colloidal particles when it is prepared by adding small amount of AgNO3 solution to KI solution in water ? What is responsible for the development of this charge ? (Comptt. Delhi 2012)
Answer:
(a) We know ΔG = ΔH – TΔS for adsorption. ΔH and ΔS are negative and ΔH > TΔS. Thus from this equation ΔG is negative. Therefore, for adsorption ΔH, ΔG and ΔS all are negative.
(b) A crystalloid can be found to behave as a colloid under a different set of conditions and vice-versa.
Example ; NaCl behaves as a crystalloid when dissolved in water but behaves as a colloid when dissolved in benzene.
(c) When AgNO3 solution is added to Kl solution, the precipitated Agl adsorbs I– ions from the dispersion medium and negatively charged colloidal solution results.
Question 73.
Explain the following:
(i) Deltas are formed when river and sea water meet.
(ii) Artificial rain is caused by spraying salt over clouds.
(iii) Physisorption is multi-layered, while chemisorption is mono-layered. (Comptt. Delhi 2012)
Answer:
(i) River water is a colloidal solution of day. Sea water contains a number of electrolytes. When river water meets the sea water, the electrolytes present in sea water coagulate the colloidal solution of clay resulting in its deposition with the formation of deltas.
(ii) Clouds are colloidal dispersion of water particles in air carrying some charge over them.
It is possible to cause artificial rain by throwing electrified sand or spraying a sol carrying charge opposite to the one on clouds from an aeroplane. The colloidal water particles present in the clouds will get neutralized and as result they will come closer and grow in size to form bigger water drops and ultimately cause artificial rain.
(iii) In physical adsorption, layers of the gas can be adsorbed one over the other by van der Waals forces. Multi-molecular layers are formed under high pressure. In chemical adsorption, chemical bond can be formed only with the layer of molecules coming in direct contact with the surface of the adsorbent, hence this type of adsorption is mono-layered.
Question 74.
How are the two types of emulsions different from one another? Give suitable examples to justify the difference. State two applications of emulsions. (Comptt. All India 2012)
Answer:
Difference between two types of emulsions are : Emulsions of oil in water in which oil is the dispersed phase and water is the dispersion medium.
Example : Milk is an emulsion of liquid fat dispersed in water.
Emulsions of water in oil in which water is the dispersed phase and oil is the dispersion medium. e.g. Cod liver oil is an emulsion of oil i.e. water is the dispersed phase and oil is the dispersion medium.
Two applications of emulsion are :
- The digestion of fats in the intestines takes place by the process of emulsification.
- Several oily drugs are prepared in the form of emulsion.
Question 75.
What is an adsorption isotherm? Describe Freundlich adsorption isotherm. (Comptt. All India 2012)
Answer:
Adsorption isotherm : The variation of the amount of the gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve. This curve is termed as adsorption isotherm at a particular temperature.
Freundlich adsorption isotherm : A mathematical equation which describes the relationship between pressure (p) of the gaseous adsorbate and the extent of adsorption at any fixed temperature is called Freundlich adsorption isotherm.
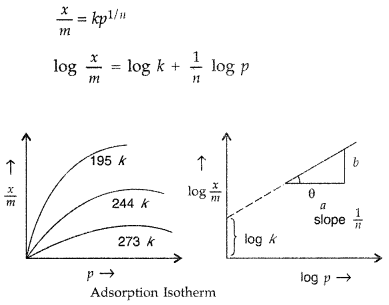
Question 76.
What are the characteristics of the following j colloids? Give one example of each.
(i) Multimolecular colloids
(ii) Lyophobic sols
(iii) Emulsions (All India 2013)
Answer:
(i) Multimolecular colloids :
- They are formed by the aggregation of a large number of atoms or molecules which generally have diameter less than 1 nm, Example : sols of gold, sulphur etc.
- Their molecular masses are not very high.
- Their atoms or molecules are held together by weak Van der Waals’ forces.
(ii) Lyophobic sols : Liquid hating colloids in which there is no affinity between disperse phase and dispersion medium.
Example: AS2S3sol. Fe(OH)3 sol.
(iii) Emulsions : The colloidal solution in which both dispersed phase and medium are in liquid state.
Example : Milk, cream.
Question 77.
Define the following terms giving an example of each :
(i) Associated colloids
(ii) Lyophilic sol
(iii) Adsorption (All India 2013)
Answer:
(i) Associated colloids :
- They are formed by aggregation of a large number of ions in concentrated solution Example : soap sol
- Their molecular masses are generally high.
- Fligher is the concentration, greater are the Vander Waals’ forces.
(ii) Lyophilic sol : Liquid loving colloids in which there is affinity between disperse phase and dispersion medium.
Example: Starch sol, Gum sol, Gelatin sol
(iii) Adsorption : The process of attracting and retaining the molecules of a substance on the surface of a liquid or a solid resulting into higher concentration of the molecules on the surface is called adsorption.
Question 78.
Define the following terms with an example in each case :
(i) Macromolecular sol
(ii) Peptization
(iii) Emulsion (All India 2013)
Answer:
(i) Macromolecular sol :
- They are molecules of large size
Example : polymers like rubber, nylon, starch proteins etc. - They have high molecular masses.
- Due to long chain, the van der Waals’ forces holding them are comparatively stronger.
(ii) Peptization : It is a process of converting a fresh precipitate into colloidal particles by shaking it with the dispersion medium in the presence of a small amount of a suitable electrolyte.
(iii) Emulsions : The colloidal solution in which both dispersed phase and medium are in liquid state.
Example : Milk, cream.
Question 79.
Explain what is observed when :
(i) A beam of light is passed through a colloidal solution.
(ii) NaCl solution is added to hydrated ferric oxide sol.
(iii) Electric current is passed through a colloidal solution. (Comptt. All India 2013)
Answer:
(i) When NaCl is added to hydrated ferric oxide solution, then coagulation will take place. A negatively charged solution is obtained with absorption of OH– ion.
(ii) When electric current is passed through a colloidal solution, the positively charged particles move towards cathode while negatively charged particles move towards anode where they lose their charge and get coagulated. The phenomenon is known as Electrophoresis.
(iii) When a beam of strong light is passed through a colloidal solution, scattering of light occurs by colloidal particles and the path of light becomes visible and the phenomenon is known as Tyndall effect.
Question 80.
(a) How can we get the following colloidal solutions :
(i) Silver in water
(ii) Fe(OH)3 in water
(b) List two applications of adsorption. (Comptt. All India 2013)
Answer:
(a) (i) Silver in water : Colloidal sol of Ag in water (Reduction with dil Sn(Cl2)
(b) Applications of adsorption :
- All gas devices contain suitable adsorbent so that poisonous gases present in the atmosphere are adsorbed and the air for breathing is purified.
- Sugar is decolourized by treating sugar solution with charcoal powder which adsorbs the undesirable colours present.
Question 81.
(a) In reference to Freundlich adsorption isotherm write the expression for adsorption of gases on solids in the form of an equation.
(b) Write an important characteristic of lyophilic sols.
(c) Based on type of particles of dispersed phase, give one example each of associated colloid and multimolecular colloid. (Delhi 2014)
Answer:
(a) The equation representing adsorption of gases on solids is
\(\frac{x}{m}\) = KP1/n
where
x / m = amount of gas adsorbed per unit mass of adsorbent
K = Constant
n = Positive integer
(b) Lyophilic sols are reversible and self stable.
(c) Associated colloid : Soap sol
Multimolecular colloid : Gold sol
Question 82.
Define the following terms:
(i) Adsorption
(ii) Peptization
(iii) Sol (Comptt. Delhi 2014)
Answer:
(i) Adsorption : It is a surface phenomenon which occurs only at the surface of the adsorbent.
(ii) Peptization : It is a process of converting a fresh precipitate into colloidal particles by shaking it with the dispersion medium in the presence of a small amount of a suitable electrolyte.
(iii) Sol : The colloids in which a solid is dispersed in the liquid, or, In a colloidal sol, the dispersed phase is a solid and the dispersion medium is a liquid.
Question 83.
Define the following terms :
(i) Sorption
(ii) Tyndall effect
(iii) Electrophoresis (Comptt. Delhi 2014)
Answer:
(i) Sorption : It is a physical and chemical process by which one substance becomes attached to another.
or, In order to distinguish between adsorption and absorption a common term sorption is used.
(ii) Tyndall effect: When a beam of light is passed through a colloidal solution and viewed perpendicular to the path of the incident light, the path of light becomes visible as a bright streak. The illuminated path is called Tyndall cone and the phenomenon is called Tyndall effect.
(iii) Electrophoresis : When electric current is passed through a colloidal solution, the positively charged particles move towards cathode while negatively charged particles move towards anode where they lose their charge and get coagulated. The phenomenon is known as Electrophoresis.
Question 84.
Giving appropriate examples, explain how the two types of processes of adsorption (physisorption and chemisorption) are influenced by the prevailing temperature, the surface area of adsorbent and the activation energy of the process? (Comptt. All India 2014)
Answer:
| Physisorption | Chemisorption |
| 1. Temperature: It decreases with increase in temperature. | 1. It increases with increase in temperature initially after adsorption it is decreasing |
| 2. Surface area of adsorbent. The adsorption area ofincreases with increase in surface area of adsorbent | 2. It also increases with inadsorbent. |
| 3. Activation energy: It requires very low or no activation energy. | 3. It requires high activation energy. |
Question 85.
Explain clearly how the phenomenon of adsorption finds application in
(i) production of vacuum in a vessel
(ii) heterogeneous catalysis
(iii) froth floatation process in metallurgy. (Comptt. All India 2014)
Answer:
(i) Production of vacuum : In Dewar flasks, activated charcoal is placed between the walls of the flask so that any gas which enters into annular space either due to glass imperfection or diffusion through glass is adsorbed and create a vacuum.
(ii) Heterogeneous catalysis : If the catalyst is present in a different phase than that of the reactants, it is called a heterogeneous catalyst and this type of catalysis is called Heterogeneous catalysis.
Example : Manufacture of NH3 from N2 and H2 by Haber’s process using iron as catalyst
N2 (g) + 3H2 (g) \(\underrightarrow { Fe\left( s \right) } \) 2NH3 (g)
Reactants are gaseous whereas catalyst is solid.
(iii) Froth Floatation process : When KCl is added to hydrated ferric oxide sol, then a negatively charged sol is obtained with absorption of OH– ion.
Question 86.
Give reasons for the following observations :
(i) Leather gets hardened after tanning.
(ii) Lyophilic sol is more stable than lyophobic sol.
(iii) It is necessary to remove CO when ammonia is prepared by Haber’s process. (Delhi 2015)
Answer:
(i) Due to mutual coagulation of leather by tanning. [Positively charged animal hyde (leather) with negatively charged colloidal particles of tannin].
(ii) Lyophilic sols are more stable because there is strong interaction between dispersed phase and dispersion medium.
(iii) Because CO acts as a poison for catalyst.
Question 87.
Write any three differences between Physisorption and Chemisorption. (All India 2015)
Answer:
| Basis | Physisorplion | Chemisorption |
| (i) Specificity | It is not specific in nature i.e. all gases are adsorbed on all solids to some extent. | It is highly specific in nature and occurs only when there is some possibility of compound formation between the gas being adsorbed and the solid being adsorbent. |
| (ii) Temperature dependence | It is independent of temperature as it occurs at low temperature and deceases with increase in temperature. | It is temperature dependent and increase with increase in temperature. |
| (iii) Reversibility | It is reversible i.e. desorption of gas takes place by increasing the temperature or decreasing the pressure. | It is irreversible in nature as it involves formation of compound instead of release of gas. |
| (iv) Enthalpy change | It has low enthalpy of adsorption i.e., 20-40 kj mol-1. | It has high enthalpy of adsorption i.e., 40-240 kj mol-1. |
Question 88.
Define the following terms :
(i) Electrophoresis
(ii) Adsorption
(iii) Shape selective catalysis (Comptt. Delhi 2015)
Answer:
(i) Electrophoresis : When electric current is passed through a colloidal solution, the positively charged particles move towards cathode while negatively charged particles move towards anode where they lose their charge and get coagulated. This phenomenon is known as electrophoresis.
(ii) Adsorption : The phenomenon of accumulation or higher concentration of molecular species (gases or , liquids) at the surface rather than in the bulk of a solid -or liquid is called adsorption.
(iii) Shape selective catalysis : The catylytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis. .
Question 89.
Describecribe the following processes:
(i) Dialysis
(ii) Tyndall effect (Comptt. All India 2015)
Answer:
(i) Dialysis : The process of removing the dissolved substance from a colloidal solution by diffusion of the mixture through a semi-permeable membrane is known as dialysis.
(ii) Tyndall effect: Colloidal particles scatter light in all directions in space. When a beam of light is passed through a colloidal solution and viewed perpendicular to the path of the incident light, the path of light becomes visible as a bright streak. The illuminated path is called Tyndall cone and the phenomenon is called Tyndall effect.
Question 90.
(i) Differentiate between adsorption and absorption.
(ii) Out of MgCl2 and AlCl3 which one is more effective in causing coagulation of negatively charged sol and why?
(iii) Out of sulphur sol and proteins, which one forms multimolecular colloids? (Delhi 2016)
Answer:
|
Adsorption |
Absorption |
| (a) It is the phenomenon by which one substance gets concentrated mainly on the surface of the other substance rather than in the bulk of a solid or liquid.
(b) It is a surface phenomenon. (c) Its concentration is different at surface from the bulk. |
(a) It is the phenomenon by which one substance gets uniformly distributed thoughout the body of the other substance.
(b) It is a bulk phenomenon. (c) Its concentration is same throughout the bulk. |
(ii) AlCl3 is more effective than MgCl2 in causing coagulation of negatively charged sol because coagulating power of an electrolyte is directly proportional to the valency of the active ion i.e., Al3+ > Mg2+.
(iii) Sulphur sol forms multimolecular colloids.
Question 91.
Define the following terms:
(i) Lyophilic colloid
(ii) Zeta potential
(iii) Associated colloids (All India 2016)
Answer:
(i) Lyophilic sol : Liquid loving colloids in which there is affinity between disperse phase and dispersion medium.
Example: Starch sol, Gum sol, Gelatin sol
(ii) Zeta potential. When one type of the ions of the electrolyte are adsorbed on the surface of colloidal particles it forms a fixed layer which attracts another layer of opposite ions thus forming a Helmholtz electrical double layer whose potential difference between the two layers is termed as zeta potential.
(iii) Associated colloids :
- They are formed by aggregation of a large number of ions in concentrated solution Example : soap sol
- Their molecular masses are generally high.
- Fligher is the concentration, greater are the Vander Waals’ forces.
Question 92.
What are emulsions? What are their different types? Give an example of each type. (Comptt. Delhi 2016)
Answer:
A liquid-liquid colloidal systems is called emulsions. They are of two types :
(i) Oil in water
(ii) Water in oil.
Examples : o/w—Vanishing cream, milk
w/ o—Butter, cold cream, cod-liver oil
Question 93.
Explain the following terms :
(i) Peptization
(ii) Lyophobic colloids
(iii) Dialysis (Comptt. All India 2016)
Answer:
(i) Peptization : The conversion of freshly prepared ppt into colloidal solution by shaking with disperson medium containing small amount of electrolyte.
(ii) Lyophobic colloids : Solvent hating colloids * are called lyophobic colloids. For example, Gold sol.
(iii) Dialysis : It is the process of removing a dissolved substance from a colloidal solution by means of diffusion through a membrane.
Question 94.
Write one difference in each of the following:
(i) Lyophobic sol and Lyophilic sol
(ii) Solution and Colloid
(iii) Homogeneous catalysis and Heterogeneous catalysis (Delhi 2017)
Answer:
(i) Lyophobic sol and Lyophilic sol. Lyophobic solutions are liquid (dispersion medium)— hating and lyophilic solutions are liquid (dispersion medium)—loving colloids.
(ii) Solution and Colloid. Solution is a homogenous solution whose particle size is less than 10-9 m while colloid is a heterogenous solution whose particle size is in between 10-9 to 10-6 m.
(iii) Homogeneous catalysis and Heterogeneous catalysis. Homogeneous catalysis is the phenomenon of changing the rate of reaction when catalyst has same phase as the reactants while in heterogeneous catalysis, the catalyst has different phase than that of the reactants.
Question 95.
Write one difference between each of the following:
(i) Multimolecular colloid and Macromolecu- lar colloid
(ii) Sol and Gel
(iii) O/W emulsion and W/O emulsion (Delhi 2017)
Answer:
(i) Multimolecular colloid. A large number of atoms or smaller molecules of a substance aggregate together to form species having size in the colloidal range.
Macromolecular colloid. Large sized molecules whose particle size lies in the colloidal range.
(ii) Sol is a colloidal system in which dispersed phase is a solid and dispersion medium is a liquid. Gel is a colloidal system in which dispersed phase is a liquid and dispersion medium is a solid.
(iii) In O/W emulsion, water acts as dispersion medium while in W/O emulsion oil acts as dispersion medium.
Question 96.
Write one difference in each of the following:
(a) Multimolecular colloid and Associated colloid
(b) Coagulation and Peptization
(c) Homogeneous catalysis and Hetero¬geneous catalysis. (All India 2017)
Answer:
(a) Multimolecular colloid and Associated colloid. Multimolecular colloids are formed by the aggregation of a large number of atoms or molecules which generally have diameters less than 1 nm, eg., sols of gold, etc. while Associated colloids are formed by the aggregation of a large number of ions in concentrated solutions, e.g., micelles in soap.
(b) Coagulation and Peptization. Coagulation is a process of aggregating together the colloidal particles into large sized particles to form their precipitate while peptization is a process of converting fresh precipitate into colloidal particles by shaking it with the dispersion medium in the presence of a small amount of a suitable electrolyte.
(c) Homogeneous catalysis and Heterogeneous catalysis. Homogeneous catalysis is the ‘ phenomenon of changing the rate of reaction when catalyst has same phase as
the reactants while in heterogeneous catalysis, the catalyst has different phase than that of the reactants.
Question 97.
(a) Write the dispersed phase and dispersion medium of milk.
(b) Write one similarity between physisorption and chemisorption.
(c) Write the chemical method by which Fe(OH)3 sol is prepared from FeCl3. (All India 2017)
Answer:
(a) In milk dispersed phase is liquid fat and dispersion medium is water.
(b) Both physisorption and chemisorption increase with increase in pressure.
(c) Fe(OH)3 is prepared from FeCl by hydrolysis.
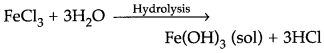
Question 98.
Define the following terms :
(i) Desorption
(ii) Critical micelle concentration
(iii) Shape selective catalysis (Comptt. Delhi 2017)
Answer:
(i) Desorption is a phenomenon whereby an adsorbed substance is removed from or through a surface.
(ii) Critical micelle concentration: The formation of micelles takes place only above a particular temperature i.e., Kraft temperature (Tk) and above a particular concentration.
(iii) Shape-selective catalysis : The catalyst reaction in which small sized molecules are absorbed in the pores and cavities of selective adsorbents like zeolites is know n as shape-selective catalysis.
Question 99.
Define the following terms :
(i) Kraft temperature
(ii) Peptization
(iii) Electrokinetic potential (Comptt. Delhi 2017)
Answer:
(i) Kraft temperature : The formation of micelles takes place only above a particular temperature known as Kraft temperature (Tk).
(ii) Peptization : It is a process of converting a fresh precipitate into colloidal particles by shaking it with the dispersion medium in the presence of a small amount of a suitable, electrolyte.
(iii) The potential difference between the fixed layer and the diffused layer is known as electrokinetic potential.
Question 100.
Explain the following phenomenon giving reasons :
(i) Tyndall effect
(ii) Brownian movement
(iii) Physical adsorption decreases with increase in temperature. (Comptt. All India 2017)
Answer:
(i) Tyndall effect: When a beam of light is passed through a colloidal solution and viewed perpendicular to the path of the incident light, the path of light becomes visible as a bright streak. The illuminated path is called^ Tyndall cone and the phenomenon is called Tyndall effect. The phenomenon is due to scattering of light by the colloidal particles. .
(ii) Brownian movement is the continuous and random zig-zag motion of the colloidal particles. This motion is due to kinetic motion of striking colloidal particles which produces a resultant force to cause motion.
(iii) Physical adsorption decreases with increase in temperature due to Le- Chatelier’s principle which shifts the equilibrium in forward direction to complete the reaction. As the adsorption is an exothermic process, it decreases with increase in temperature.