Important Questions for Class 12 Chemistry Chapter 3 Electrochemistry Class 12 Important Questions
Electrochemistry Class 12 Important Questions Very Short Answer Type
Question 1.
What is meant by ‘limiting molar conductivity’? (All India 2010)
Answer:
The molar conductivity of a solution at infinite dilution is called limiting molar conductivity and is represented by the symbol Λm.
Question 2.
Express the relation between conductivity and molar conductivity of a solution held in a cell. (Delhi 2011)
Answer:
Λm = \(\frac{\mathrm{K}}{\mathrm{C}}=\frac{\text { Conductivity }}{\text { Concentration }}\)
Question 3.
What is the effect of catalyst on:
(i) Gibbs energy (ΔG) and
(ii) activation energy of a reaction? (Delhi 2017)
Answer:
(i) There will be no effect of catalyst on Gibbs .energy.
(ii) The catalyst provides an alternative pathway by decreasing the activation energy of a reaction.
Question 4.
What is the effect of adding a catalyst on
(a) Activation energy (Ea), and
(b) Gibbs energy (AG) of a reaction? (All India 2017)
Answer:
(a) On adding catalyst in a reaction, the activation energy reduces and rate of reaction is fastened.
(b) A catalyst does not alter Gibbs energy (AG) of a reaction.
Electrochemistry Class 12 Important Questions Short Answer Type -I [SA – I]
Question 5.
Two half cell reactions of an electrochemical cell are given below :
MnO–4(aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I), E° = + 1.51 V
Sn2+ (aq) → 4 Sn4+ (aq) + 2e–, E° = + 0.15 V
Construct the redox equation from the two half cell reactions and predict if this reaction favours formation of reactants or product shown in the equation. (All India 2009)
Answer:
The reactions can be represented at anode and at cathode in the following ways :
At anode (oxidation) :
Sn2+ → = Sn4+ (aq) + 2e– ] × 5 E° = + 0.15 V
At cathode (reduction) :
MnO–4(aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I)] × 2 E° = + 1.51 V
The Net R × M = 2MnO–4(aq) + 16H+ + 5Sn2+ → 2Mn2+ + 5Sn4+ + 8H2O
Now E°cell = E°cathode – E°anode
= 1.51 – 0.15 = + 1.36 V
∴ Positive value of E°cell favours formation of product.
Question 6.
Express the relation among the cell constant, the resistance of the solution in the cell and the conductivity of the solution. How is the conductivity of a solution related to its molar conductivity? (All India 2010)
Answer:
\(\frac{1}{\mathrm{R}} \times \frac{1}{a}\) = Conductance (C) × Cell constant
Molar conductance : (Λm) = \(\frac{\mathrm{K} \times 1000}{\mathrm{c}}\).
Question 7.
Given that the standard electrode potentials (E°) of metals are :
K+/K = -2.93 V, Ag+/Ag = 0.80 V, Cu2+/Cu = 0.34 V,
Mg2+/Mg = -2.37 V, Cr3+/Cr = -0.74 V, Fe2+/Fe = -0.44 V.
Arrange these metals in increasing order of their reducing power. (All India 2010)
Answer:
Ag+/Ag < Cu2+/Cu < Fe2+/Fe < Cr3+/Cr < Mg2+/ Mg < K+/K
More negative the value of standard electrode potentials of metals is, more will be the reducing power.
Question 8.
Two half-reactions of an electrochemical cell are given below :
MnO–4 (aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I), E° = 1.51 V
Sn2+ (aq) → Sn4+ (aq) + 2e–, E° = + 0.15 V.
Construct the redox reaction equation from the two half-reactions and calculate the cell potential from the standard potentials and predict if the reaction is reactant or product favoured. (All India 2010)
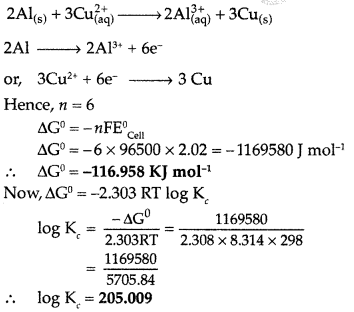
Answer:
The reactions can be represented at anode and at cathode in the following ways :
At anode (oxidation) :
Sn2+ → Sn4+ (aq) + 2e– ] × 5 E° = + 0.15 V
Af cathode (reduction) :
MnO–4(aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I)] × 2 E° = + 1.51 V
The Net R × M = 2MnO–4(aq) + 16H+ + 5Sn2+ → 2Mn2+ + 5Sn4+ + 8H2O
Now E°cell = E°cathode – E°anode
= 1.51 – 0.15 = + 1.36 V
∴ Positive value of E°cell favours formation of product.
Question 9.
The chemistry of corrosion of iron is essentially an electrochemical phenomenon. Explain the reactions occurring during the corrosion of iron in the atmosphere. (Delhi 2011)
Answer:
The mechanism of corrosion is explained on the basis of electrochemical theory. By taking example of rusting of iron, we Refer tothe formation of small electrochemical cells on the surface of iron.
The redox reaction involves
At anode : Fe(S) → Fe2+ (aq) + 2e–
At cathode : H2O + CO2 ⇌ H2CO3 (Carbonic acid)
H2CO3 ⇌2H+ + CO22-
H2O ⇌ H+ + OH–
H+ + e– → H
4H + O2 → 2H2O
Then net resultant Redox reaction is
2Fe(s) + O2 (g) + 4H+ → 2Fe2+ + 2H2O
Question 10.
Determine the values of equilibrium constant (Kc) and ΔG° for the following reaction :
Ni(s) + 2Ag+ (aq) → Ni2+ (aq) + 2Ag(s),
E° = 1.05 V
(1F = 96500 C mol-1) (Delhi 2011)
Answer:
According to the formula
ΔG° = -nFE° = – 2 × 96500 ×1.05
or ΔG° = -202650 J mol-1 = -202.65 KJ mol-1
Now ΔG° ⇒ -202650 J Mol-1
R = 8.314 J/Mol/K, T = 298 K
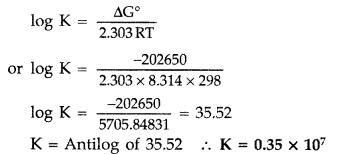
Question 11.
Two half-reactions of an electrochemical cell are given below :
MnO–4 (aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I), E° = 1.51 V
Sn2+ (aq) → Sn4+ (aq) + 2e–, E° = + 0.15 V.
Construct the redox equation from the standard potential of the cell and predict if the reaction is reactant favoured or product favoured. (Delhi 2011)
Answer:
The reactions can be represented at anode and at cathode in the following ways :
At anode (oxidation) :
Sn2+ → = Sn4+ (aq) + 2e– ] × 5 E° = + 0.15 V
At cathode (reduction) :
MnO–4(aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I)] × 2 E° = + 1.51 V
The Net R × M = 2MnO–4(aq) + 16H+ + 5Sn2+ → 2Mn2+ + 5Sn4+ + 8H2O
Now E°cell = E°cathode – E°anode
= 1.51 – 0.15 = + 1.36 V
∴ Positive value of E°cell favours formation of product.
Question 12.
Express the relation among cell constant, resistance of the solution in the cell and conductivity of the solution. How is molar conductivity of a solution related to its conductivity? (All India 2012)
Answer:
GG* = K
where Q is conductance;
G * is cell constant;
K is conductivity
G* × \(\frac{1}{\mathrm{R}}\) = K ⇒ G* = RK
∴ Λm = \(\frac{\mathbf{K} \times 1000}{\mathbf{C}}\) S cm2 mol-1
Question 13.
The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm2 mol-1. Calculate the conductivity of this solution. (All India 2012)
Answer:
C = 1.5 M, Λm = 138.9 S cm2 mol-1
Λm = \(\frac{\mathrm{K} \times 1000}{\mathrm{c}}\)
∴K = \(\frac{\Lambda_{m} \times C}{1000}=\frac{138.9 \times 1.5}{1000}\) = 0.20835 S cm-1
Question 14.
A zinc rod is dipped in 0.1 M solution of ZnSO4. The salt is 95% dissociated at this dilution at 298 K. Calculate the electrode potential.
[ E°Zn2+ /Zn = – 0.76 V] (Comptt. Delhi 2012)
Answer:
The electode reaction is given as
Zn+2 + 2e → Zn
Using Nernest Equation
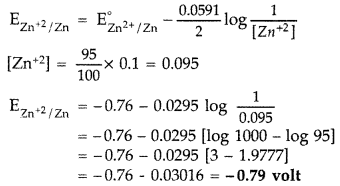
Question 15.
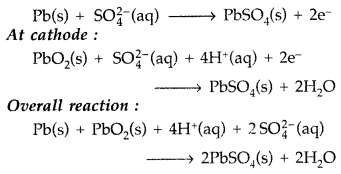
Write the reactions taking place at cathode and anode in lead storage battery when the battery is in use. What happens on charging the battery ? (Comptt. All India 2012)
Answer:
At Anode: Pb + SO4-2 → PbSO4 + 2e
at Cathode : PbO2 + SO4-2 + 4H+ + 2e → PbSO4 + 2H2O
On charging the battery, the reaction is reversed and PbSO4 on anode and cathode is converted into Pb and PbO2 respectively.
Question 16.
The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm-1. Calculate its molar conductivity. (Delhi 2013)
Answer:
Molar conductivity Λm = \(\frac{1000 \times \kappa}{M}\)
Given : K = 0.025 S cm-1, M = 0.20 M
Hence, Λm = \(\frac{0.025 \times 1000}{0.20}\) ∴ Λm = 125 S cm2 mol-1
Question 17.
The standard electrode potential (E°) for Daniel cell is +1.1 V. Calculate the ΔG° for the reaction
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
(1 F = 96500 C mol-1). (All India 2013)
Answer:
We know, ΔG° = -nF E°cell
Given : E°cell = 1.1 volt
∴ ΔG° = -2 × 96500 C mol-1 × 1.1 volt
= -212300 CV mol-1
= -212300 J mol-1 = -212.3 KJ mol-1
Question 18.
The conductivity of 0.001 M acetic acid is 4 × 10-5 S/cm. Calculate the dissociation constant of acetic acid, if molar conductivity at infinite dilution for acetic acid is 390 S cm2/mol. (Comptt. Delhi 2013)
Answer:
Given : K = 4 × 10-5 S/cm, M = 0.001 M
Λ°m = 390 S cm2/mol, k = ?
Using the formula
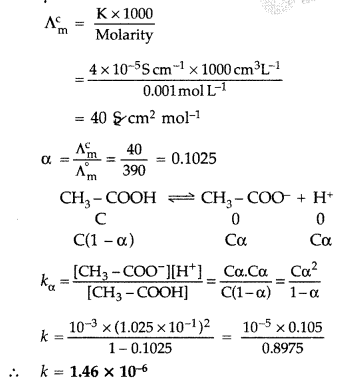
Question 19.
The standard electrode potential for Daniell cell is 1.1 V. Calculate the standard Gibbs energy for the cell reaction. (F = 96,500 C mol-1) (Comptt. Delhi 2013)
Answer:
Given : E° = 1.1V, F = 96,500 C mol-5, n = 2
Zn + Cu2 ⇌ Cu + Zn2+
Using ΔG° = -nFE° = -2 × 96500 × 1.1
= 212,300 CV mol-1
Question 20.
State Kohlrausch law of independent migration of ions. Why does the conductivity of a solution decrease with dilution? (All India 2014)
Answer:
Kohlrausch law of independent migration of ions: The limiting molar conductivity of an electrolyte (i.e. molar conductivity at infinite dilution) is the sum of the limiting ionic conductivities of the cation and the anion each multiplied with the number of ions present in one formula unit of the electrolyte
Λ°m for AxBy = xλ°+ + yλ°–
For acetic acid Λ° (CH3COOH) = λ°CH3COO– + λ°H+
Λ°(CH3COOH) = Λ° (CH3COOK) + Λ° (HCl) – Λ° (KCl)
Question 21.
Define the following terms :
(i) Fuel cell
(ii) Limiting molar conductivity (Λ°m) (All India 2014)
Answer:
(i) Fuel cells : These cells are the devices which convert the energy produced during combustion of fuels like H2, CH4, etc. directly into electrical energy.
(ii) The molar conductivity of a solution at infinite dilution is called limiting molar conductivity and is represented by the symbol Λ°m.
Question 22.
Define the following terms :
(i) Molar conductivity (Λm)
(ii) Secondary batteries (All India 2014)
Answer:
Molar conductivity: Molar conductivity of a solution at a given concentration is the conductance of the volume ‘V’ of a solution containing one mole of electrolyte kept between two electrodes with area of cross section ‘A’ and distance of unit length. It is represented by Λm (lamda).
Λm = \(\frac{\mathrm{KA}}{l}\) I = 1 and A = V
∴ Λm = KV Unit = S cm2 mol-1
Secondary batteries : Those batteries which can be recharged by passing an electric current through them and can be used again and again are called secondary batteries.
Question 23.
Define the following terms :
(i) Rate constant (k)
(ii) Activation energy (Ea) (Comptt. Delhi 2014)
Answer:
(i) Rate constant (k): It is a proportionality constant and is equal to the rate of reaction when the molar concentration of each of the reactants is unity.
(ii) Activation energy (Ea): The minimum extra amount of energy absorbed by the reactant molecules to form the activated complex is called activation energy.
Question 24.
Set up Nemst equation for the standard dry cell. Using this equation show that the voltage of a dry cell has to decrease with use. (Comptt. All India 2014)
Answer:
Cell reaction of a dry cell can be represented as
Zn + Hg2+ → Zn2+ + Hg (n = 2)
Nemst equation
Ecell = E°cell – \(\frac{0.0591}{2} \log \frac{\left[\mathrm{Zn}^{2+}\right]}{\mathrm{Hg}^{2+}}\)
The voltage of dry cell has to decrease because the concentration of electrolyte decreases in the reactions.
Question 25.
Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their Variation with change in temperature. (Comptt. All India 2014)
Answer:
Conductivity: Conductivity of a solution is defined as the conductance of a solution of 1 cm length and having 1 sq. cm as the area of cross-section. It is represented by K.
Its unit is S cm-1
Molar conductivity : Molar conductivity of a solution at a dilution V is the conductance of all the ions produced from one mole of the electrolyte dissolved in V cm3 of the solution when the electrodes are 1 cm apart and the area of the electrodes is so large that the whole of the solution is contained between them. It is represented by Λm.
Its unit is S cm2 mol-1
Conductivity and molar conductivity of electrolytes increase with increasing temperature.
Question 26.
(a) Following reactions occur at cathode during the electrolysis of aqueous silver chloride solution :
Ag+(aq) + e– → Ag(s) E° = +0.80 V
H+(aq) + e– → \(\frac{1}{2}\)H2(g) E° = 0.00 V
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why?
(b) Define limiting molar conductivity. Why conductivity of an electrolyte solution decreases with the decrease in concentration? (Delhi 2015)
Answer:
(a) At the cathode Ag+ (aq) + e– → Ag(s)
reaction is feasible, because Ag+ ion has higher reduction potential i.e. higher E° value.
(b) Limiting molar conductivity or the molar conductivity of solution at infinite dilution is the sum of molar conductivity cations and anions.
Conductivity of an electrolyte solution decreases on dilution because number of ions per unit volume decreases.
Question 27.
Calculate the time to deposit 1.27 g of copper at cathode when a current of 2A was passed through the solution of CuSO4.
(Molar mass of Cu = 63.5 g mol-1,1 F = 96500 C mol-1) (All India 2015)
Answer:
CuSO4 → Cu+ + SO42-
Cu2+ + 2e– → Cu
63.5 gram of copper is deposited = 2 × 96500 C
1.27 gram of Cu is deposited = \(\frac{2 \times 96500}{63.5}\) × 1.27
= I × t (Q = I × t)
t = \(\frac{2 \times 96500 \times 1.27}{63.5 \times 2}\) = 1930 seconds
Question 28.
From the given cells: Lead storage cell, Mercury cell, Fuel cell and Dry cell
Answer the following:
(i) Which cell is used in hearing aids?
(ii) Which cell was used in Apollo Space Programme?
(iii) Which cell is used in automobiles and inverters?
(iv) Which cell does not have long life?(Delhi 2016)
Answer:
(i) Mercury cell is used in hearing aids.
(ii) Fuel cell was used in the Apollo Space Programme.
(iii) Lead storage cell is used in automobiles and inverters.
(iv) Dry cell does not have a long life.
Question 29.
Calculate the degree of dissociation (a) of acetic acid if its molar conductivity (Λm) is 39.05 S cm2 mol-1.
Given: λ°(H+) = 349.6 S cm2 mol-1 and λ°(CH3COO–) = 40.9 S cm2 mol-1 (Delhi 2017)
Answer:
Λ°m(HAC) = λ°H+ + λ°AC–
= λ°CH3COOH = λ°H+ + λ°CH3COO–
= 349.6 S cm2 mol-1 + 40.9 S cm2 mol-1
= 390.5 S cm2 mol-1
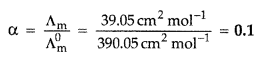
Question 30.
Write the name of the cell which is generally used in hearing aids. Write the reactions taking place at the anode and the cathode of this cell. (All India 2017)
Answer:
Mercury cells are used in hearing aids.
Reaction at anode:
Zn (Hg) + 2OH– → ZnO (s) + H2O + 2e–
Reaction at cathode:
HgO + H2O + 2e– → Hg (l) + 2OH–
Question 31.
Write the name of the cell which is generally used in transistors. Write the reactions taking place at the anode and the cathode of this cell. (All India 2017)
Answer:
Leclanche cells (Dry cell) is used in transistors.
Reaction at Anode:
Zn(s) → Zn2+ + 2e–
At Cathode:
MnO2 + \(\mathrm{NH}_{4}^{+}\) + e– → MnO(OH) + NH3
Question 32.
Write the name of the cell which is generally used in inverters. Write the reactions taking place at the anode and the cathode of this cell. (Delhi 2017)
Answer:
Lead storage battery is used in inverters.
At Anode:
Pb(s) + \(\mathrm{SO}_{4}^{2-}(\mathrm{aq})\) → PbSO4 (s) + 2e–
At Cathode:
PbO2(s) + \(\mathrm{SO}_{4}^{2-}(\mathrm{aq})\) + 4H+ (aq) + 2e–
PbSO4 (s) + 2H2O
Question 33.
Following reactions can occur at cathode during the electrolysis of aqueous silver nitrate solution using Pt electrodes :
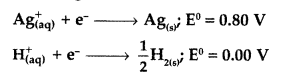
On the basis of their standard electrode potential values, which reaction is feasible at cathode and why? (Comptt. All India 2017)
Answer:
As the standard electrode potential of silver is greater than that of the other hydrogen electrode, so reduction of silver takes place and reaction (i) will be feasible
i.e., \(\mathrm{Ag}_{(\mathrm{aq})}^{+}\) + e– → Ag(s)
Question 34.
Following reactions may occur at cathode during the electrolysis of aqueous silver nitrate solution using silver electrodes :
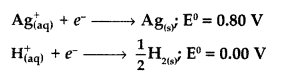
On the basis of their standard electrode potential values, which reaction is feasible at cathode and why? (Comptt. All India 2017)
Answer:
The value of \(\mathrm{E}_{\mathrm{cell}}^{0}\) is positive due to higher standard electrode potential (E° = +0.80 V) of Ag+ than that of H+ so reaction (i) will be feasible at cathode
i.e. \(\mathrm{Ag}_{(\mathrm{aq})}^{+}\) + e– → Ag(s). Silver has higher reduction potential.
Question 35.
Following reactions may occur at cathode during the electrolysis of aqueous CuCl2 solution using Pt electrodes:
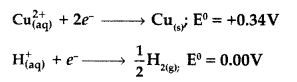
On the basis of their standard electrode potential values, which reaction is feasible at cathode and why? (Comptt. All India 2017)
Answer:
Since the standard electrode potential of Cu2+ is greater than that of H+, so reaction (i) will be feasible at cathod
i.e. \(\mathrm{Cu}_{(\mathrm{aq})}^{2+}\) + 2e → Cu
Cu2+ has higher reduction potential.
Electrochemistry Class 12 Important Questions Short Answer Type -II [SA – II]
Question 36.
A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential is measured 0,422 V. Determine the concentration of silver ion in the cell.
Given : E°Ag+/Ag = + 0.80 V, E° Cu2+/Cu = + 0.34 V. (All India 2009)
Answer:
The reaction takes place at anode and cathode in the following ways :
At anode (oxidation) :
Cu(s) → Cu2+(aq) + 2e–
At cathode (reduction) :
Cu(s) + 2Ag2+(aq) → Cu2+(aq) + 2Ag(s)
The complete cell reaction is

Question 37.
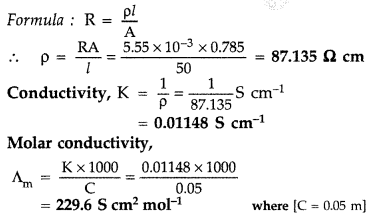
The electrical resistance of a column of 0.05 M NaOH solution of diameter 1 cm and length 50 cm is 5.55 × 103 ohm. Calculate its resistivity, conductivity and molar conductivity.(All India 2012)
Answer:
A = πr2 = 3.14 × (0.5)2 = 0.785 cm2, l = 50 cm
Question 38.
A voltaic cell is set up at 25°C with the following half cells :
Al/Al3+ (0.001 M) and Ni/Ni2+ (0.50 M)
Write an equation for the reaction that occurs when the cell generates an electric current and determine the cell potential.
\(E_{\mathrm{Ni}^{2+} / \mathrm{Ni}}^{0}=-0.25 \mathrm{V} \text { and } E_{\mathrm{Al}^{3+} / \mathrm{Al}}^{0}=-1.66 \mathrm{V}\)
(Log 8 × 10-6 = -0.54) (All India 2012)
Answer:
Half cell reactions and overall cell reaction are
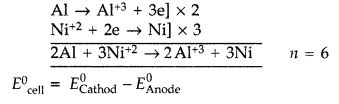
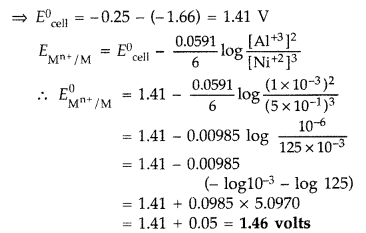
Question 39.
A solution containing 30 g of non-volatile solute exactly in 90 g of water has a vapour pressure of 2.8 kPa at 298 K. Further 18 g of water is added to this solution. The new vapour pressure becomes 2.9 kPa at 298 K. Calculate
(i) the molecular mass of solute and
(ii) vapour pressure of water at 298 K. (Comptt. Delhi 2012)
Answer:
For a very dilute solution
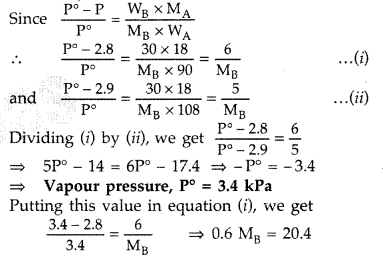
∴ Molecular mass, MB = 34 g/mol.
Question 40.
What is corrosion? Explain the electrochemical theory of rusting of iron and write the reactions involved in the rusting of iron. (Comptt. Delhi 2012)
Answer:
Corrosion: Corrosion is defined as the deterioration of a substance because of its reaction with its environment. Corrosion is an electrochemical phenomenon. At a particular spot of an object made of iron, oxidation takes place and that spot behaves as anode and the reaction is
At Anode : 2Fe → 2Fe+2 + 4e–
Electrons released at anodic spot move through the metal and go to another spot on the metal and reduce oxygen in presence of H+. This spot behaves as cathode
At Cathode : O2 + 4H+ + 4e-
Overall reaction : 2Fe + O2 + 4H+ → 2Fe+2 + 2H2O
Question 41.
When a certain conductance cell was filled with 0.1 M KCl, it has a resistance of 85 ohms at 25°C. When the same cell was filled with an aqueous solution of 0.052 M unknown electrolyte, the resistance was 96 ohms. Calculate the molar conductance of the electrolyte at this concentration.
[Specific conductance of 0.1 M KCl = 1.29 × 10-2 ohm-1 cm-1] (Comptt. All India 2012)
Answer:
Cell contant = Conductivity × Resistance
G* = K × R
= 1.29 × 10-2Ω-1 × 85
= 109.65 × 10-2Ω-1
= 1.0965 cm-1
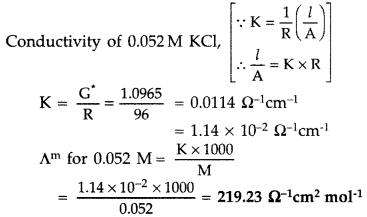
Question 42.
(a) How many coulombs are required to reduce 1 mole Cr2O72- to Cr3+?
(b) The conductivity of 0.001 M acetic acid is 4 × 10-5 S/m. Calculate the dissociation constant of acetic acid if \(\Lambda_{\mathrm{m}}^{0}\),for acetic acid is 390 S cm2 mol-1. (Comptt. All India 2012)
Answer:
(a) Cr2O7-2 + 14H+ + 6e → 2Cr-3 + 7H2O
∴ 6 Faraday of charge is required
(b) Conductivity (K) = 4 × 10-5 S cm-1
Concentration (C) = 0.001M
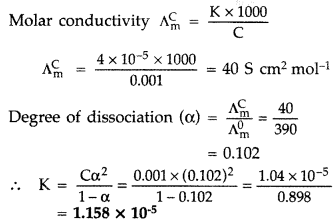
Question 43.
The cell in which the following reaction occurs :
2Fe3+ (aq) + 2I– (aq) → 2Fe2+ (aq) + I2 (s) has E0cell = 0.236V at 298K. Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
(Antilog of 6.5 = 3.162 × 106; of 8.0 = 10 × 108; of 8.5 = 3.162 × 108) (Comptt. All India 2012)
Answer:
log KC = \(\frac{n E_{\text { cell }}^{0}}{0.0591}=\frac{2 \times 0.236}{0.0591}\) = 8
KC = antilog 8 = 1 × 108
ΔG° = -nFE0cell = -2 × 96500 × 0.236
= -45548 J/mol-1
= -45.548 kJ/mol-1
Question 44.
Calculate the emf of the following cell at 298 K: Fe(s) | Fe2+ (0.001 M) || H+ (1M) | H2(g) (1 bar), Pt(s) (Given E°cell = +0.44V) (Delhi 2013)
Answer:
As Fe + 2H+ → Fe2+ + H2 (n = 2)
According to Nernst equation
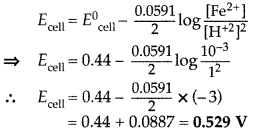
Question 45.
Calculate the emf of the following cell at 25°C : Ag(s) | Ag+ (10-3 M) || Cu2+ (10-1 M) | Cu(s) Given E0cell = +0.46 V and log 10n = n. (All India 2013)
Answer:
Given: Cell notation is incorrect. Correct cell formula is
Cu2+ (10-1 m) | Cu(5) || Ag+ (10-3 M) | Ag(s)
According to Nernst equation
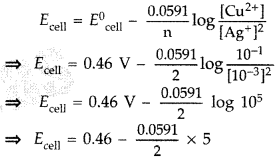
∴Ecell = 0.46 V – 0.14775 = 031 V
Question 46.
(a) What are fuel cells? Explain the electrode reactions involved in the working of H2 – O2 fuel cell.
(b) Represent the galvanic cell in which the reaction
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) takes place. (Comptt. Delhi, Comptt. All India 2013)
Answer:
(a) Fuel cells : These cells are the devices which convert the energy produced during combustion of fuels like H2, CH4, etc. directly into electrical energy.
The electrode reaction for H2 – O2 fuel cell :
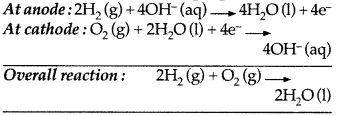
(b) The galvanic cell may be represented as Zn (s) | Zn2+ (1M) || Cu2+ (1M) | Cu (s)
Question 47.
(a) State and explain Kohlrausch law.
(b) How much electricity in terms of Faradays is required to produce 20 g of calcium from molten CaCl2? (Comptt. Delhi 2013)
Answer:
(a) Corrosion is an electrochemical phenomenon. At a particular spot of iron j oxidation it takes place and the spot behaves j as anode and the reaction will be: i
At anode :
2Fe(s) → 2Fe2+ + 4e–
Electrons released above move through the metal to another spot and reduce oxygen in presence of H+ and the spot behaves as cathode with the reaction.
At cathode :
O2(g) + 4H+ (aq) + 4e– → 2H2O(I)
Overall reaction :
2Fe(s) + O2(g) + H+(aq) → 2Fe2+(aq) + 2H2O (l)
The ferrous ions are further oxidised by atmospheric oxygen to form hydrated ferric oxide (Fe2O3 xH2O) as rust.
(b) Ca22+ + 2e– → Ca
Thus, 1 mole of Ca i.e. 40 g of Ca requires electricity = 2F
∴ 20 g of Ca will require electricity = 1F
Question 48.
Silver is uniformly electro-deposited on a metallic vessel of surface area of 900 cm2 by passing a current of 0.5 ampere for 2 hours. Calculate the thickness of silver deposited.
[Given: the density of silver is 10.5 g cm-3 and atomic mass of Ag = 108 amu.] (Comptt. All India 2013)
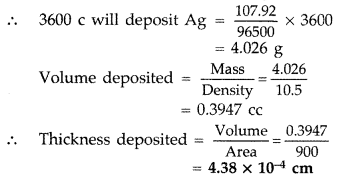
Answer:Quantity of electricity passed
= 0.5 × 2 × 60 × 60 = 3600 c
Ag+ + e– → Ag
96500 c deposits Ag = 107.92 g
Question 49.
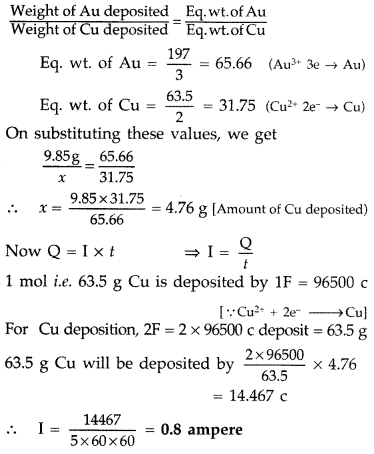
A current was passed for 5 hours through two electrolytic cells connected in series. The first cell contains AuCl3 and second cell CuSO4 solution. If 9.85 g of gold was deposited in the first cell, what amount of copper gets deposited in the second cell? Also calculate magnitude of current in ampere.
Given: Atomic mass of Au = 197 amu and Cu = 63.5 amu. (Comptt. All India 2013)
Answer:
Question 50.
(a) Calculate ΔrG0 for the reaction
Mg (s) + Cu2+ (aq) → Mg2+ (aq) + Cu (s)
Given : E0cell = + 2.71 V, 1 F = 96500 C mol-1
(b) Name the type of cell which was used in Apollo space programme for providing electrical power. (All India 2014)
Answer:
(a) ΔrG0 = – nFE0
= -2 × 96500 × 2.71 (∵ n = 2)
= -523,030 J mol-1 = -523.03 KJ mol-1
(b) Fuel cell was used in Apollo space programme for providing electrical power.
Question 51.
The resistance of 0.01 M NaCl solution at 25° C is 200 Ω. The cell constant of the conductivity cell used is unity. Calculate the molar conductivity of the solution.(Comptt. All India 2014)
Answer:
For 0.01 M NaCl solution,
R = 200 Ω, cell constant is unity.
∴ Conductivity (K) = \(\frac{\text { Cell constant }}{\text { Resistance }}\)
= \(\frac{1}{200}\) = 0.005 Sm-1
Concentration of solution = 0.01 M = 0.01 mol L-1
= 0.01 × 103 mol m-3 = 10 mol m-3
Molar conductivity = \(\frac{K}{C_{m}}=\frac{0.005}{10}\)
5 × 10-4 Sm2 mol-1
Question 52.
Calculate emf of the following cell at 25°C :
Fe | Fe2+ (0.001 M) || H+ (0.01 M) | H2(g) (1 bar) | Pt(s)
E0(Fe2+ | Fe) = -0.44 V E0(H+ | H2) = 0.00V (Delhi 2015)
Answer:
Fe | Fe2+ (0.001 M) || H+ (0.01 M) | H2(g) (1 bar) | Pt(s)
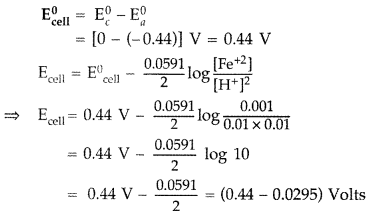
= 0.4105 Volts
Question 53.
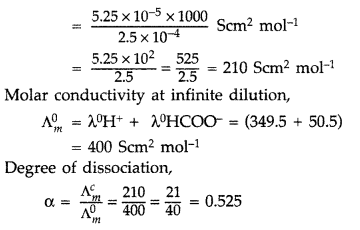
Conductivity of 2.5 × 10-4 M methanoic acid is 5.25 × 10-5 S cm-1. Calculate its molar conductivity and degree of dissociation.
Given : λ0(H+) = 349.5 Scm2 mol-1
and λ0(HCOO–) = 50.5 Scm2 mol-1. (All India 2015)
Answer:
Concentration is 2.5 x 10-4 M
K = 5.25 × 10-5 Scm-1.
\(\Lambda_{m}^{c}=\frac{\mathrm{K} \times 1000}{\text { Concentration }}\)
Question 54.
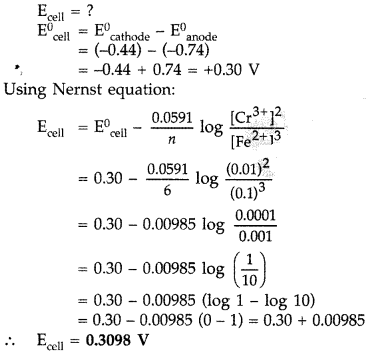
Calculate e.m.f. of the following cell at 298 K: 2Cr(s) + 3Fe2+ (0.1 M) → 2Cr3+ (0.01 M) + 3 Fe(s)
Given: E0(Cr3+| Cr) = -0.74 V E0(Fe2+ | Fe) = -0.44 V (Delhi 2016)
Answer:
Cell reaction: 2Cr(s) + 3Fe2+ (0.1 M) → 2Cr3+ (0.01 M) + 3Fe(s)
Given: E0(Cr3+/Cr) = -0.74
E0(Fe2+/Fe) = -0.44 V
Question 55.
(i) Calculate the mass of Ag deposited at cathode when a current of 2 amperes was passed through a solution of AgNO3 for 15 minutes.
[Given: Molar mass of Ag = 108 g mol-1 1F = 96,500 C mol-1)
(ii) Define fuel cell. (Delhi 2017)
Answer:
(i) Q = I × t …(Charge = Current ∝ Time)
. = 2 × 15 × 60 = 1800 C
∵ 96500 C deposit Ag = 108 g
∴ 1800 C deposit Ag = \(\frac{108}{96500}\) × 1800
= 2.0145 g
(ii) Cells that convert the energy of combustion of fuels like hydrogen, methanol, methane, etc directly into electrical energy are called fuel cells.
Question 56.
(a) The cell in which the following reaction occurs:
2Fe3+ (aq) + 2I–(aq) → 2Fe2+ (aq) + I2(s)
has \(\mathbf{E}_{\text { Cell }}^{\mathrm{o}}\) = 0.236 V at 298 K. Calculate the standard Gibbs energy of the cell reaction. (Given: 1F = 96,500 C mol-1)
(b) How many electrons flow through a metallic wire if a current of 0.5 A is passed for 2 hours? (Given: 1F = 96,500 C mol-1) (All India 2017)
Answer:
(a) 2Fe3+ (aq) + 2I– (aq) → 2Fe2+ (aq) + I2 (s)
For the given reaction, n = 2, E° = 0.236 V
Using formula
ΔG° = -nF E°cell
= -2 × 96,500 C mol-1 × 0.236 V
∴ ΔG° =-45.55 kj mol-1
(b) Given:
I = 0.5 A
t = 2 hrs. = 2 × 60 ×60 s = 7,200 s
Q = I × t = 0.5 × 7200 = 3,600 C
96,500 C electricity flows to produce
= 6.022 × 1023 electrons
∴ 1 C electricity flows to produce

Question 57.
Why an electrochemical cell stop working after some time? The reduction potential of an electrode depends upon the concentration of solution with which it is in contact. (All India 2017)
Answer:
As the cell works, the concentration of reactants decrease. Then according to Le chatelier’s pri¬nciple it will shift the equilibrium in backward direction. On the other hand if the concentration is more on the reactant side then it will shift the equilibrium in forward direction. When cell works concentration in anodic compartment in cathodic compartment decreases and hence E° cathode will decrease. Now EMF of cell is
E0cell = E0cathode – E0anode
A decrease in E°cathode and a corresponding increase in E°anode will mean that EMF of the cell will decrease and will ultimately become zero i.e., cell slops working after some time.
Question 58.
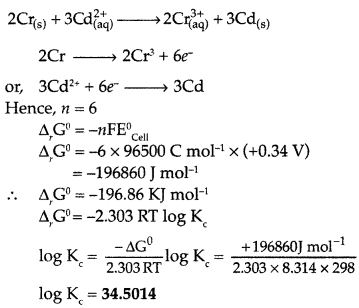
Calculate ΔrG° and log Kc for the following reaction at 298 K.
\(2 \mathrm{Cr}_{(\mathrm{s})}+3 \mathrm{Cd}_{(\mathrm{aq})}^{2+} \longrightarrow 2 \mathrm{Cr}_{\mathrm{raq}}^{3+}+3 \mathrm{Cd}_{(\mathrm{s})}\)
[Given : \(\mathbf{E}_{\mathrm{Cell}}^{\mathbf{0}}\) = +0.34 V, IF = 96500 C mol-1] (Comptt. All India 2017)
Answer:
Question 59.
Calculate ΔrG° and log K. for the following reaction at 298 K. (Comptt. All India 2017)
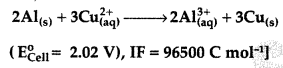
Answer:
Electrochemistry Class 12 Important Questions long Answer Type (LA)
Question 60.
(a) Define molar conductivity of a substance and describe how for weak and strong electrolytes, molar conductivity changes with concentration of solute. How is such change explained?
(b) A voltaic cell is set up at 25°C with the following half cells :
Ag+ (0.001 M) | Ag and Cu2+ (0.10 M) | Cu What would be the voltage of this cell? (E0cell = 0.46 V) (Delhi 2009)
Answer:
(a) Molar conductivity: Molar conductivity of a solution at a given concentration is tire conductance of the volume ‘V’ of a solution containing one mole of electrolyte kept between two electrodes with area of cross section ‘A’ and distance of unit length. It is represented by Λm (lamda).
Λm = \(\frac{K A}{l}\) ∴ l = 1 and A = V
∴ Λm= KV Unit = S cm2 mol-1
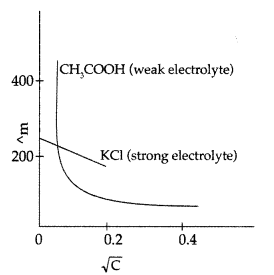
Effect of change of concentrations on molar conductivity. In case of strong electrolytes there is a small increase in conductance with dilution because a strong electrolyte is completely dissociated in solution and the number of ions remains constant. Moreover there will be greater inter-ionic attractions at higher concentrations which retards the motion of ions and conductance decreases. In case of weak electrolytes there is increase in conductance with decrease in concentration due to the increase in the number of ions in the solution.
The graph between Λm and concentration also rectifies the above statement.
(b) The reaction takes place in cell as
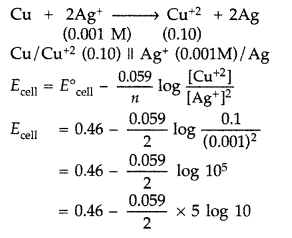
= 0.46 – \(\frac{0.059}{2}\) × 5
= 0.46 – 0.1475 = 0.3125
Question 61.
(a) State the relationship amongst cell constant of a cell, resistance of the solution in the cell and conductivity of the solution. How is molar conductivity of a solute related to conductivity of its solution?
(b) A voltaic cell is set up at 25°C with the following half-cells :
Al | Al3+ (0.001 M) and Ni | Ni2+ (0.50 M)
Calculate the cell voltage
\(E_{\mathrm{Ni}^{2+} / \mathrm{Ni}}^{0}=-0.25 \mathrm{V}, E_{\mathrm{Ni}^{2+} / \mathrm{Ni}}^{0}=-1.66 \mathrm{V}\)
(Delhi 2009)
Answer:
(a) The relationship between the cell constant of a cell (G*), resistance of the solution in the cell (R) and conductivity (K) is given by
K = \(\frac{\text { Cell constant }}{\mathrm{R}}=\frac{\mathrm{G}^{\star}}{\mathrm{R}}\)
The relationship between molar conductivity (Λm) and conductivity of the solution (K) is given by
Λm = \(\frac{\mathrm{K}}{\mathrm{C}}\)
(b) The cell may be represented as
Al | Al3+ || Ni2+| Ni
E0cell = E0R – E0L
E0cell = (-0-25) – (-1.66)
∴ E0 = -0.25 + 1.66 = 1.41 V
Question 62.
(a) What type of a cell is the lead storage battery? Write the anode and the cathode reactions and the overall reaction occurring in a lead storage battery while operating.
(Delhi, All India 2009)
(b) A voltaic cell is set up at 25 °C with the half-cells, Al | Al3+ (0.001 M) and Ni | Ni2+ (0.50 M). Write the equation for the reaction that occurs when the cell generates an electric current and determine the cell potential.
(Given : \(E_{\mathrm{Ni}^{2+} / \mathrm{Ni}}^{\circ}=-0.25 \mathrm{V}, E_{\mathrm{Al}^{3+} / \mathrm{Al}}^{\circ}=-1.66 \mathrm{V}\)) (Delhi 2009)
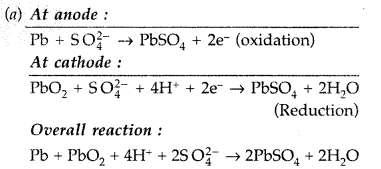
Answer:
(a) The lead storage battery is a secondary cell (rechargeable). During discharging the electrode reaction occurs as follows :
At anode :
(b) The cell may be represented as
Al | Al3+ || Ni2+| Ni
E0cell = E0R – E0L
E0cell = (-0-25) – (-1.66)
∴ E0 = -0.25 + 1.66 = 1.41 V
Question 63.
(a) Express the relationship amongst cell constant, resistance of the solution in the cell and conductivity of the solution. How is molar conductivity of a solute related to conductivity of its solution?
(b) Calculate the equilibrium constant for the reaction
Fe(s) + Cd2+(aq) ⇌ Fe2+(aq) + Cd(s)
(Given : \(E_{\mathrm{Cd}^{2+} / \mathrm{Cd}}^{0}\) = -0.40 V,
\(E_{\mathrm{Cd}^{2+} / \mathrm{Cd}}^{0}\) = -0.44 V). (Delhi 2009)
Answer:
(a) The relationship between the cell constant of a cell (G*), resistance of the solution in the cell (R) and conductivity (K) is given by
K = \(\frac{\text { Cell constant }}{\mathrm{R}}=\frac{\mathrm{G}^{\star}}{\mathrm{R}}\)
The relationship between molar conductivity (Λm) and conductivity of the solution (K) is given by
Λm = \(\frac{\mathrm{K}}{\mathrm{C}}\)
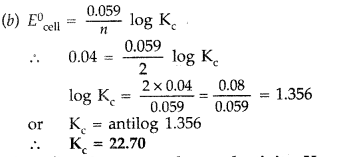
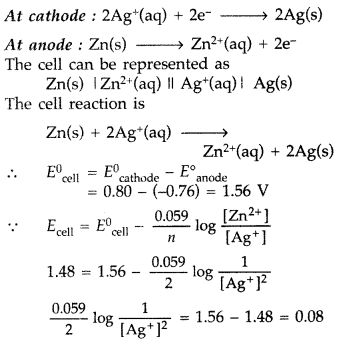
Question 64.
(a) Define the term molar conductivity. How is it related to conductivity of the related solution?
(b) One half-cell in a voltaic cell is constructed from a silver wire dipped in silver nitrate solution of unknown concentration. Its other half-cell consists of a zinc electrode dipping in 1.0 M solution of Zn(NO3)2. A voltage of 1.48 V is measured for this cell. Use this information to calculate the concentration of silver nitrate solution used.
(\(E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{0}=-0.76 \mathrm{V}, E_{\mathrm{Ag}^{+} / \mathrm{Ag}}^{0}\) = + 0.80 V) (Delhi 2009)
Answer:

(b) Half cell reactions :
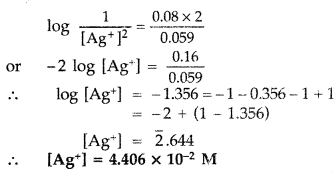
Question 65.
(a) Corrosion is essentially an electrochemical phenomenon. Explain the reactions ; occurring during corrosion of iron kept in j an open atmosphere.
(b) Calculate the equilibrium constant for the equilibrium reaction
Fe(s) + Cd2+(aq) ⇌ Fe2+(aq) + Cd(s)
(Given : \(E_{\mathrm{Cd}^{2+} / \mathrm{Cd}}^{0}\) = – 0.40 V,
\(E_{\mathrm{Fe}^{2+} / \mathrm{Fe}}^{0}\) = -0-44V) (Delhi 2009)
Answer:
(a) Corrosion is an electrochemical i phenomenon. At a particular spot of iron j oxidation it takes place and the spot behaves j as anode and the reaction will be : i
At anode :
2Fe(s) → 2Fe2+ + 4e–
Electrons released above move through the metal to another spot and reduce oxygen in presence of H+ and the spot behaves as cathode with the reaction.
At cathode :
O2(g) + 4H+ (aq) + 4e– → 2H2O(I)
Overall reaction :
2Fe(s) + O2(g) + H+(aq) → 2Fe2+(aq) + 2H2O (l)
The ferrous ions are further oxidized by atmospheric oxygen to form hydrated ferric oxide (Fe2O3 xH2O) as rust
Question 66.
(a) State Kohlrausch law of independent migration of ions. Write an expression for the molar conductivity of acetic acid at infinite dilution according to Kohlrausch law.
(b) Calculate Λ°m for acetic acid.
Given that Λ°m (HCl) = 426 S cm2 mol-1
Λ°m (NaCl) = 126 S cm2 mol-1
Λ°m (CH3COONa) = 91 S cm2 mol-1 (Delhi 2010)
Answer:
(a) Kohlrausch law of independent migration of ions : The limiting molar conductivity of an electrolyte (i.e. molar conductivity at infinite dilution) is the sum of the limiting ionic conductivities of the cation and the anion each multiplied with the number of ions present in one formula unit of the electrolyte
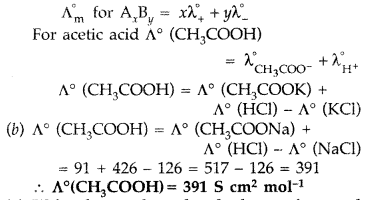
Question 67.
(a) Write the anode and cathode reactions and the overall reaction occuring in a lead storage battery.
(b) A copper-silver cell is set up. The copper ion concentration is 0.10 M. The concen¬tration of silver ion is not known. The cell potential when measured was 0.422 V. Determine the concentration of silver ions in the cell. (Delhi 2010)
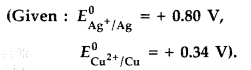
Answer:
(b) The reaction takes place at anode and cathode in the following ways :
At anode (oxidation) :
Cu(s) → Cu2+(aq) + 2e–
At cathode (reduction) :
Cu(s) + 2Ag2+(aq) → Cu2+(aq) + 2Ag(s)
The complete cell reaction is

Question 68.
(a) What type of a battery is lead storage battery? Write the anode and cathode reactions and the overall cell reaction occuring in the operation of a lead storage battery.
(b) Calculate the potential for half-cell containing
0.10 M K2Cr2O7 (aq), 0.20 M Cr3+ (aq) and 1.0 × 10-4 M H+ (aq).
The half-cell reaction is
Cr2O72-(aq) + 14 H+ (aq) + 6e– → 2 Cr3+ (aq) + 7 H2O (l)
and the standard electrode potential is given as E0 = 1.33 V. (All India 2011)
Answer:
(a) The lead storage battery is a secondary cell (rechargeable). During discharging the electrode reaction occurs as follows :
At anode :

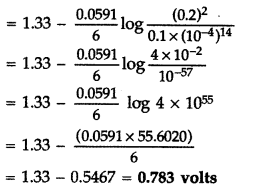
(b) E = ? E0 = 1.33 V
E0cell = 1.33 – \(\frac{0.0591}{6} \log \frac{\left[\mathrm{Cr}^{+3}\right]^{2}}{\left[\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\right]\left[\mathrm{H}^{+}\right]^{14}}\)
Question 69.
(a) How many moles of mercury will be produced by electrolysing 1.0 M Hg(NO3)2 solution with a current of 2.00 A for 3 hours? [Hg(NO3)2 = 200.6 g mol-1]
(b) A voltaic cell is set up at 25 °C with the following half-cells Al2+ (0.001 M) and Ni2+ (0.50 M). Write an equation for the reaction that occurs when the cell generates an electric current and determine the cell potential.
(Given : \(E_{\mathrm{Ni}^{2+} / \mathrm{Ni}}^{0}\) = – 0.25 V, \(E_{\mathrm{Al}^{3+} / \mathrm{Al}}^{0}\) = – 1.66 V) (All India 2011)
Answer:
(a) Quantity of electricity (Q) = I × t
= 2 × 3 × 60 × 60
= 21600 C
Hg2+ + 2e– → Hg
Thus 2F i.e. 2 × 96500 C deposists Hg = 1 mole
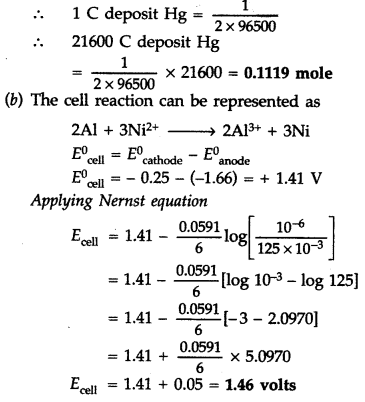
Question 70.
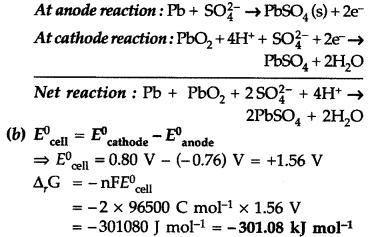
(a) What type of a battery is the lead storage battery? Write the anode and the cathode reactions and the overall reaction occurring in a lead storage battery when current is drawn from it
(b) In the button cell, widely used in watches, the following reaction takes place
Zn(s) + Ag2O(s) → Zn2+(aq) + 2Ag(s) + 2OH–O(aq)
Determine E0 and ΔG0 for the reaction.
(Given \(E_{\mathrm{A} \mathrm{g}^{+} / \mathrm{Ag}}^{0}\) = +0.80V, \(E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{0}\) = -0.76 V) (Delhi 2011)
Answer:
(a) It is a secondary cell.
Question 71.
(a) Define molar conductivity of a solution and explain how molar conductivity changes with change in concentration of solution for a weak and a strong electrolyte.
(b) The resistance of a conductivity cell containing 0.001 M KCl solution at 298 K is 1500 Ω. What is the cell constant if the conductivity of 0.001 M KCl solution at 298 K is 0.146 × 10-3 S cm-1 ? (Delhi 2011)
Answer:
(a) Molar conductivity: Conductivity of 1 M electrolytic solution placed between two electrodes 1 cm apart and have enough area of cross-section to hold the entire volume is known as molar conductivity or conductivity observed for one molar solution of electrolyte. Molar condctivity increases with decrease in concentration of solute for both weak and strong electrolytes.
(b) R = ρ (l/a)
Cell constant \(\frac{l}{a}=\frac{R}{\rho}\) = RK
= (1500 Ω) × 0.146 × 10-3 S cm-1 = 0.219 cm-1
Question 72.
(a) Define the following terms :
(i) Limiting molar conductivity (ii) Fuel cell
(b) Resistance of a conductivity cell filled with 0.1 mol L-1 KCl solution is 100 Ω. If the resistance of the same cell when filled with 0.02 mol L-1 KCl solution is 520 Ω, calculate the conductivity and molar conductivity of 0.02 mol L-1 KCl solution. The conductivity of 0.1 mol L-1 KCl solution is 1.29 × 10-2 Ω-1 cm-1. (Delhi 2014)
Answer:
(a) (i) Fuel cells : These cells are the devices which convert the energy produced during combustion of fuels like H2, CH4, etc. directly into electrical energy.
(ii) The molar conductivity of a solution at infinite dilution is called limiting molar conductivity and is represented by the symbol Λ°m.
(b) For 0.1 M KCl solution
R = 100 Q, K = 1.29 × 10-2 Ω-1 cm-1
Formula : Cell constant
= Conductivity × Resistance
= 1.29 × 10-2 × 100
= 1.29 cm-1 or 129 m-1
For 0.2 M KCl solution, conductivity
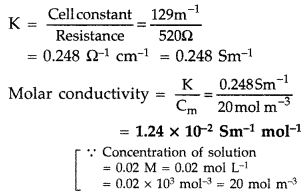
Question 73.
(a) State Faraday’s first law of electrolysis. How much charge in terms of Faraday is required for the reduction of 1 mol of Cu2+ to Cu.
(b) Calculate emf of the following cell at 298 K : Mg(s) | Mg2+ (0.1 M) || Cu2+ (0.01) | Cu (s)
[Given E0cell = +2.71 V, 1 F = 96500 C mol-1] (Delhi 2014)
Answer:
According to first law of Faraday’s “the amount of chemical reaction and hence the mass of any substance deposited/liberated at any electrode is directly proportional to the quantity of electricity passed through the electrolyte.”
The quantity of charge required for reduction of 1 mol of Cu2+
= 2 faradays (∵ Cu2+ + 2e– → Cu)
= 2 × 96500 C = 193000 C
Cell reaction : Mg + Cu2+ Mg2+ + Cu(n = 2)
Using Nernst equation,
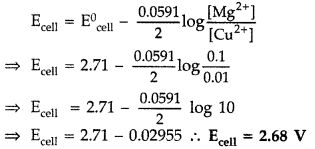
Question 74.
(a) Define the terms conductivity and molar conductivity for the solution of an electrolyte. Comment on their variation with temperature.
The measured resistance of a conductance cell was 100 ohms. Calculate (i) the specific conductance and (ii) the molar conductance of the solution.
(KC1 = 74.5 g mol-1 and cell constant = 1.25 cm-1 ) (Comptt. Delhi 2014)
Answer:
(a) Conductivity : Reciprocal of resistivity is called conductivity
k = \(\frac{1}{R} \times \frac{l}{A}\)
Molar conductivity : It is defined as the conductivity of solution containing 1 mole solute dissolved per litre when placed between two electrodes of unit area separated by 1 cm.
Molar conductivity and conductivity of solution increase on increasing the temperature.
(b) Given : R = 100 Ω Cell constant = 1.25 cm-1
Molarity = 74.5 g mol-1
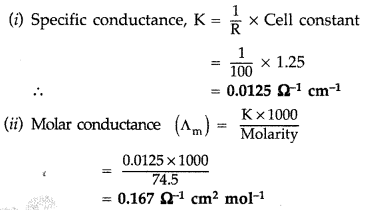
Question 75.
(a) Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with platinum electrodes.
(ii) An aqueous solution of H2SO4 with platinum electrodes.
(b) Estimate the minimum potential difference needed to reduce Al2O3 at 500°C. The Gibbs energy change for the decomposition reaction \(\frac{2}{3}\) Al2O3 → \(\frac{4}{3}\) Al + O2 is 960 kJ
(F = 96500 C mol-1) (Comptt. Delhi 2014)
Answer:
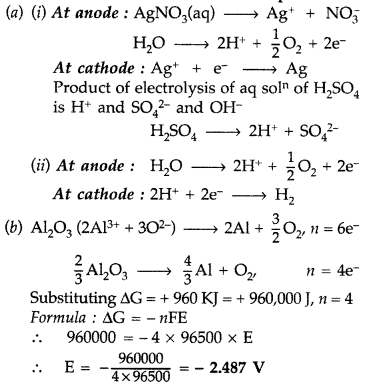
Question 76.
(a) Define the following terms :
(i) Molar conductivity (Λm)
(ii) Secondary batteries
(iii) Fuel cell
(b) State the following laws :
(i) Faraday first law of electrolysis
(ii) Kohlrausch’s law of independent migration of ions (Comptt. Delhi 2014)
Answer:
(a) (i) Molar conductivity Λm): Molar conductivity can be defined as the conductance of the volume V of electrolytic solution kept between two electrodes of a conducting cell at distance of unit length but having area of cross section large enough to accomodate sufficient volume of solution that contains one mole of the electrolyte.
Λm = KV
(ii) Secondary batteries: Those cells which can be recharged on passing electric current through them in opposite direction and can be used again are called secondary batteries, e.g. Lead-acid storage cell.
(iii) Fuel cell : Galvanic cells that are designed to convert the chemical energy of combustion of fuels like hydrogen, methane etc. into electrical energy are called fuel cells, e.g. H2 – O2 fuel cell
(b) (i) Faraday first law of electrolysis : According to this law the mass of the substance deposited or liberated at any electrode during electrolysis is directly proportional to the quantity of charge passed through the electrolyte.
ω ∝ Q (∵ Q = I × t)
ω = = ZIt
(ii) Kohlrausch’s law of independent migration of ions : According to this law limiting molar conductivity of an electrolyte can be represented as the sum of the limiting ionic conductivities of the cation and the anion each multiplied with the number of ions present in one formula unit of electrolyte.
\(\Lambda_{m}^{\mathrm{o}} \text { for } \mathrm{A} x \mathrm{B} y=x \lambda_{+}^{\mathrm{o}}+y \lambda_{-}^{\mathrm{o}}\)
Question 77.
(a) Define the term degree of dissociation. Write an expression that relates the molar conductivity of a weak electrolyte to its degree of dissociation.
(b) For the cell reaction
Ni(s) | Ni2+(aq) || Ag+(aq) | Ag(s)
Calculate the equilibrium constant at 25 °C. How much maximum work would be obtained by operation of this cell?
\(\mathrm{E}_{\mathrm{Ni}^{2} / \mathrm{Ni}}^{\mathrm{o}}\) = 0.25 V and \(\mathbf{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}^{\mathrm{o}}\) = 0.80 V (Comptt. Delhi 2014)
Answer:
(a) Degree of dissociation: It is the measure of the extent to which an electrolyte gets dissociated into its constitutent ions.
Thus higher the degree of dissociation, higher will be its molar conductance. Mathematically it can be expressed as :
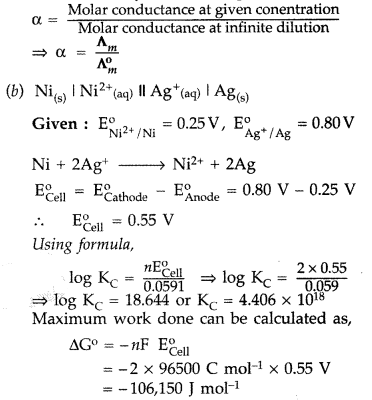
Maximum work = 106.150 KJ mol-1
Question 78.
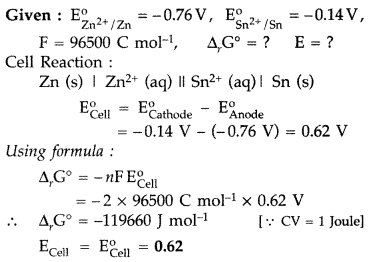
Calculate ΔrG° and e.m.f. (E) that can be obtained from the following cell under the standard conditions at 25°C :
Zn (s) | Zn2+ (aq) || Sn2+ (aq) | Sn (s)
Answer:
Question 79.
(a) Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
(b) Calculate the standard cell potential of the galvanic cell in which the following reaction takes place :
Fe2+ (aq) + Ag+ (aq) → Fe3+ (aq) + Ag (s) Calculate the ΔrG° and equilibrium constant of the reaction also.
(\(\mathbf{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}^{0}\) = 0.80 V; \(\mathbf{E}_{\mathrm{Fe}^{3+} / \mathrm{Fe}^{2+}}^{0}\) = 0.77 V)
(Comptt. All India 2014)
Answer:
(a) Conductivity : The conductance of the solution of an electrolyte enclosed in a cell between two electrodes of unit area of cross section separated by 1 cm. It is represented as K with unit ohm-1 cm-1
Molar conductivity : It is the conductance of the volume V of solution containing one mole of electrolyte kept between two electrodes with area of cross section A and distance of unit length.
Molar conductivity increases with decrease in concentration of solute for both weak and strong electrolytes.
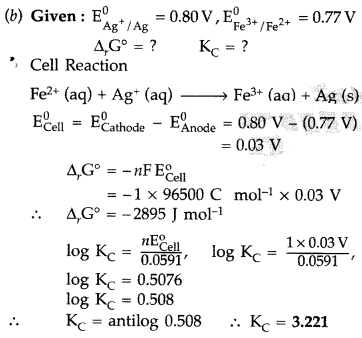
Question 80.
(a) Calculate E0cell for the following reaction at 298 K:
2Al(s) + 3Cu2+ (0.01M) → 2Al2+ (0.01M) + 3Cu(s)
Given: Ecell = 1.98 V
(b) Using the E0 values of A and B, predict which is better for coating the surface of iron [E0(Fe2+/Fe) = -0.44 V] to prevent corrosion and why?
Given: E0(A2+/A) = -2.37 V; E0(B2+/B) = -0.14 V (All India 2016)
Answer:
(a) For the reaction
2Al(s) + 3Cu2+ (0.01M) → 2Al3+ (0.01M) + 3Cu(s)
Given: Ecell = 1.98 V E0cell = ?
Using Nernst equation
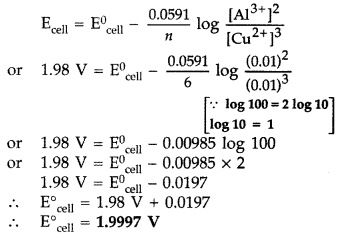
(b) Element A will be better for coating the surface of iron than element B because its E° value is more negative.
Question 81.
(a) The conductivity of 0.001 mol L-1 solution of CH3COOH is 3.905 × 10-5 S cm-1. Calculate its molar conductivity and degree of dissociation (α).
Given: λ0(H+) = 349.6 S cm2 mol-1 and λ0 (CH3COO–) = 40.9 S cm2 mol-1
(b) Define electrochemical cell. What happens if external potential applied becomes greater than E0cell of electrochemical cell? (All India 2016)
Answer:
(a) Concentration = 0.001 mol L-1
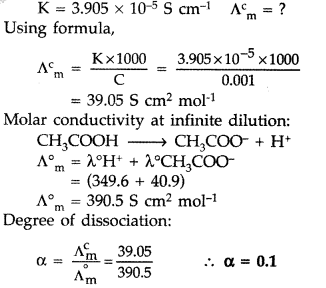
(b) Electrochemical cell: It is a device which converts chemical energy into electrical energy i.e., produced as a result of redox reaction taking place in the electrolyte.
The reaction gets reversed and it becomes non-spontaneous. It starts acting as an electrolytic cell.
Question 82.
(a) Define the following :
(i) Molar conductivity
(ii) Fuel cell
(b) The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm2 mol-1 Calculate the conductivity of the solution. (Comptt. Delhi 2016)
answer:
(a) (i) Molar conductivity Λm): Molar conductivity can be defined as the conductance of the volume V of electrolytic solution kept between two electrodes of a conducting cell at distance of unit length but having area of cross section large enough to accomodate sufficient volume of solution that contains one mole of the electrolyte.
Λm = KV
(ii) Secondary batteries: Those cells which can be recharged on passing electric current through them in opposite direction and can be used again are called secondary batteries, e.g. Lead-acid storage cell.
(iii) Fuel cell : Galvanic cells that are designed to convert the chemical energy of combustion of fuels like hydrogen, methane etc. into electrical energy are called fuel cells, e.g. H2 – O2 fuel cell
(b) Λm = \(\frac{k \times 1000}{c}\) S cm2 mol-1
138.9 = \(\frac{k \times 1000}{1.5}\)
k = 0.208 S cm-1
Question 83.
(a) Define the following terms :
(i) Primary batteries
(ii) Corrosion
(b) The resistance of a conductivity cell containing 0.001 M KCl solution at 298 K is 1500 Ω. What is the cell constant if conductivity of 0.001 M KCl solution at 298 K is 0.146 × 10-3 S cm-1? (Comptt. Delhi 2016)
Answer:
(a) (i) Primary batteries : Batteries which can’t be rechared/reused.
(ii) Corrosion : Loss of useful metals due to oxidation on exposure to atmospheric gases and moisture.
(b) k = \(\frac{1}{R}\left(\frac{l}{A}\right)\)
\(\frac{l}{\mathrm{A}}\) = k × R = 0.146 × 10-3 S cm-1 × 1500 Ω
\(\frac{l}{\mathrm{A}}\) = 0.219 cm-1
Question 84.
(a) What are the two classifications of batteries? What is the difference between them?
(b) The resistance of 0.01 M NaCl solution at 25°C is 200 Ω. The cell constant of the conductivity cell is unity. Calculate the molar conductivity of the solution. (Comptt. All India 2016)
Answer:
(a) Two types of batteries are Primary batteries and Secondary batteries. Primary batteries are non-chargeable whereas secondary batteries are rechargeable.
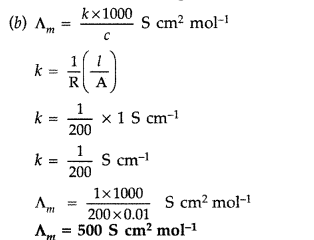
Question 85.
(a) What are fuel cells? Give an example of a fuel cell.
(b) Calculate the equilibrium constant (log Kc) and ΔrG° for the following reaction at 298 K.
Cu (s) + 2Ag+ (aq) ⇌ Cu2+ (aq) + 2Ag (s)
Given E0cell = 0.46 V and IF = 96500 C mol-1 (Comptt. All India 2016)
Answer:
(a) Cell which converts energy of combustion of fuel directly into electricity.
Example : H2 – O2 fuel cell.
Or
Those cells which convert fuel energy directly into electrical energy.
Example : H2 – O2 fuel cell
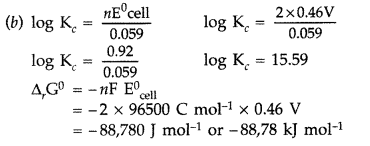
Question 86.
(a) When a bright silver object is placed in the solution of gold chloride, it acquires a golden tinge but nothing happens when it is placed in a solution of copper chloride. Explain this behaviour of silver.
[Given : \(\mathbf{E}_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{0}\) =+0.34V,\(\mathbf{E}_{\mathbf{A} \mathbf{g}^{+} / \mathbf{A g}}^{\mathbf{0}}\) =+0.80V, \(\mathrm{E}_{\mathrm{Au}^{3+} / \mathrm{Au}}^{0}\) = +1.40V]
(b) Consider the figure given and answer the following questions :
(i) What is the direction of flow of electrons?
(ii) Which is anode and which is cathode?
(iii) What will happen if the salt bridge is removed?
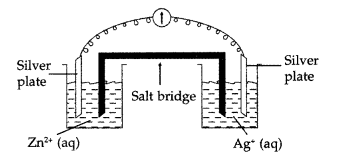
(iv) How will concentration of Zn2+ and Ag+ ions be affected when the cell functions?
(v) How will concentration of these ions be affected when the cell becomes dead? (Comptt. Delhi 2017)
Answer:
(a) The standard electrode potential, E° for silver is 0.80 V and that of gold is 1.5 V, hence silver can displace gold from its solution. The replaced gold is deposited on silver object due to which golden tinge is obtained. On the other hand E° for Cu is 0.34 V which is lower than that of silver, thus silver cannot replace copper from its solution.
(b)
(i) Electrons flow from anode (Zinc plate) to cathode (Silver plate).
(ii) Zinc plate where oxidation occurs acts as anode and silver plate where reduction occurs acts as cathode.
(iii) If the salt bridge is removed then electrons from zinc electrode will flow to the silver electrode where they will neutralize some of Ag+ ions and the \(\mathrm{SO}_{4}^{2-}\) ions will be left and the solution will acquire a negative charge. Secondly the Zn2+ ions from zinc plate will enter into ZnSO4 solution producing positive charge. Thus due to accumulation of charges in two solutions, further flow of electrons will stop and hence the current stops flowing and the cell will stop functioning.
(iv) As silver from silver sulphate solution is deposited on the silver electrode and sulphate ions migrate to the other side, the concentration of AgSO4 solution decreases and of ZnSO4 solution increases as the cell operates.
(v) When the cell becomes dead, the concentration of these ions become equal due to attainment of equilibrium and zero EMF.
Question 87.
(a) What is limiting molar conductivity? Why there is steep rise in the molar conductivity of weak electrolyte on dilution?
(b) Calculate the emf of the following cell at 298 K :
Mg (s) | Mg2+ (0.1 M) || Cu2+ (1.0 × 10-3M) | Cu (s)
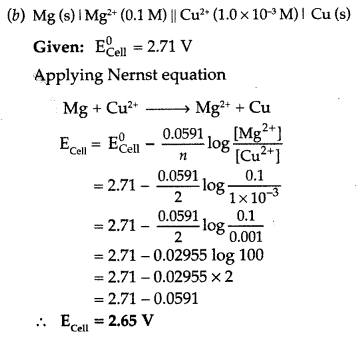
[Given = \(\mathbf{E}_{\text { Cell }}^{0}\) = 2.71 V]. (Comptt. Delhi 2017)
Answer:
(a) The molar conductivity of a solution at infinite dilution is called limiting molar conductivity and is represented by the
symbol \(\Lambda_{m}^{\circ}\)
There is steep rise in the molar conductivity of weak electrolyte on dilution because as the concentration of the weak electrolyte is reduced, more of it ionizes and thus increase in the number of ions in the solution.