Important Questions for Class 12 Chemistry Chapter 16 Chemistry in Everyday Life Class 12 Important Questions
Chemistry in Everyday Life Class 12 Important Questions Very Short Answer Type
Question 1.
Differentiate between disinfectants and antiseptics. (Delhi 2012)
Answer:
| Antiseptics | Disinfectants |
| 1. They are chemical substances which prevent the growth of micro-organisms and may even kill them. | 1. They are chemical substances which kill micro-organisms. |
| 2. They are safe to be applied to the living tissues. | 2. They are not safe to be applied to the living tissues. |
| 3. They are generally applied on wounds, cuts, ulcers and diseased skin surfaces. Example : Furacin, soframycin, dettol and savlon, 0.2% solution of phenol. | 3. They are used to kill micro-organisms present in the drains, toilets, floors etc. Example: Phenol (> 1% solution) and chlorine (0.2 to 0.4 ppm). |
Question 2.
What are limited spectrum antibiotics? Give one example. (Comptt. Delhi 2012)
Answer:
Those antibiotics which are specific for certain diseases are called limited spectrum antibiotics. Example: Streptomycin for tuberculosis.
Question 3.
Name the important by-products of soap industry. (Comptt. Delhi 2012)
Answer:
Glycerol is the important by-product of soap industry.
Question 4.
Why do we require artificial sweetening agents? (Comptt. All India 2012)
Answer:
To reduce calorie intake and to protect teeth from decaying, we need artificial sweetners.
Chemistry in Everyday Life Class 12 Important Questions Short Answer Type SA-I
Question 5.
What are food preservatives? Name two such substances. (All India 2012)
Answer:
Food preservatives : Food preservatives are the compounds which prevent spoilage of food due to microbial growth.
Two substances : Example : Sodium benzoate, vinegar.
Question 6.
Explain the cleaning action of soap. Why do soaps not work in hard water? (All India 2012)
Answer:
Cleaning action of soap : The cleansing action of soap is due to the fact that soap molecules form micelles around the oil droplets in such a way that hydrophobic part of stearate ions is in the oil droplet and hydrophilic part projects out of the grease droplet like the bristles. Since the polar groups can interact with water, the oil droplet surrounded by stearate ions is now pulled in water and removed from the dirty surface. Thus soap helps in emulsification and washing away of oils and fats.
Reason : Hard water contains calcium and magnesium ions. These ions form insoluble Ca and Mg salts. These salts act as scum. The insoluble scum sticks on the clothes, so the cleaning capacity of soap is reduced when Na or K soaps are dissolved in hard water.
Question 7.
Explain the following terms with suitable examples :
(a) Cationic detergents
(b) Anionic detergents (Comptt. Delhi 2013)
Answer:
(a) Cationic detergents :
(i) Cationic detergents : They are quaternary ammonium salts of amines with acetates, chlorides or bromides as anions and the cationic part possess a long hydrocarbon chain, and a positive charge on nitrogen atom. Therefore they are called cationic detergents.
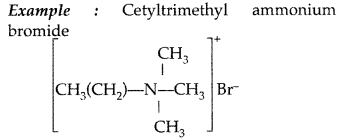
(b) Anionic detergents : Those detergents in which large part of their molecules are anions and used in cleansing action, are called anionic detergents.
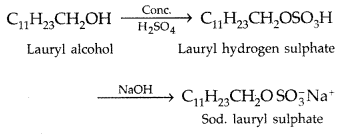
Example : Sodium alkyl sulphates These are obtained from long straight chain alcohols containing 12-18 carbon atoms by treatment with cone. H2S04 followed by neutralization with NaOH.
Example : Sodium lauryl sulphate.
Chemistry in Everyday Life Class 12 Important Questions Short Answer Type SA-II
Question 8.
Explain the following types of substances with one suitable example, for each case :
(i) Cationic detergents.
(ii) Food preservatives.
(iii) Analgesics. (Delhi 2009)
Answer:
(i) Cationic detergents : They are quaternary ammonium salts of amines with acetates, chlorides or bromides as anions and the cationic part possess a long hydrocarbon chain, and a positive charge on nitrogen atom. Therefore they are called cationic detergents.

(ii) Food preservatives : They are used to prevent spoilage of food due to microbial growth.
Example : Table salt, vegetable oils, sodium benzoate etc.
(iii) Analgesics : Analgesics reduce or abolish pain without causing impairment of consciousness, mental confusion, in coordination or paralysis or some other disturbance of nervous system.
They are of two types :
(a) Non-narcotic analgesics Example : Aspirin
(b) Narcotic analgesics Example : Morphine
Question 9.
How do antiseptics differ from disinfectants? Give one example of each type. (Delhi 2008)
Antiseptics Disinfectants
Answer:
| Antiseptics | Disinfectants |
| 1. They are chemical substances which prevent the growth of micro-organisms and may even kill them. | 1. They are chemical substances which kill micro-organisms. |
| 2. They are safe to be applied to the living tissues. | 2. They are not safe to be applied to the living tissues. |
| 3. They are generally applied on wounds, cuts, ulcers and diseased skin surfaces. Example : Furacin, soframycin, dettol and savlon, 0.2% solution of phenol. | 3. They are used to kill micro-organisms present in the drains, toilets, floors etc. Example: Phenol (> 1% solution) and chlorine (0.2 to 0.4 ppm). |
Question 10.
What are the following substances? Give one example of each type.
(i) Antacid
(ii) Non-ionic detergents
(iii) Antiseptics (All India 2008)
Answer:
(i) Antacid : Those substances which neutralize the excess acid and raise the pH to an appropriate level in stomach are called antacids.
Example : Sodium bicarbonate, Mg(OH)2
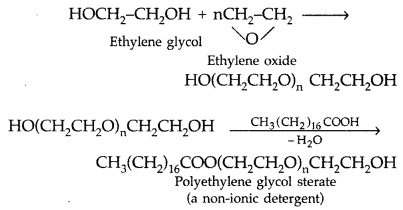
(ii) Non-ionic detergents : These are esters of high molecular mass alcohols obtained by reaction between polyethylene glycol and steric acid.
Example :
(iii) Antiseptics : These are chemical substances which prevent the growth of micro-organisms and may even kill them and safe to be applied on living tissues.
Example : Furacin, soframycin etc.
Question 11.
Describe the following substances with one suitable example of each type : (All India 2009)
(i) Non-ionic detergents
(ii) Food preservatives
(iii) Disinfectants
Answer:
(i) Non-ionic detergents : These are esters of high molecular mass alcohols obtained by reaction between polyethylene glycol and steric acid.
Example :

(ii) Food preservatives : They are used to prevent spoilage of food due to microbial growth.
Example : Table salt, vegetable oils, sodium benzoate etc.
(iii) Disinfectants :
Disinfectants are chemical compounds which kill microorganisms but are not safe when applied on living organisms.
Example : Phenol, chlorine.
Question 12.
What are the following substances? Give one example of each of them. (All India 2009)
(i) Cationic detergents
(ii) Enzymes
(iii) Sweetening agents
Answer:
(i) Cationic detergents : They are quaternary ammonium salts of amines with acetates, chlorides or bromides as anions and the cationic part possess a long hydrocarbon chain, and a positive charge on nitrogen atom. Therefore they are called cationic detergents.

(ii) Enzymes : Enzymes are biological catalysts which are chemically globular proteins having high molecular mass and highly specific in their actions due to presence of active sites of definite shape and size on their surfaces so that only specific substrate can fit in them.
Example : Pepsin, amylase
(iii) Sweetening agents : Those chemical substances which are sweet in taste but do not add any calories to our body are called artificial sweetening agents. These are excreted as such through urine.
Example : Saccharin, aspartame etc.
Question 13.
What are analgesic medicines? How are they classified and when are they commonly recommended for use? (Delhi 2010)
Answer:
Analgesic medicine : Drugs which reduce or abolish pain without causing reduction of consciousness, mental confusion, incoordination or paralysis or some other disorder of the nervous system are called analgesic medicines.
They are classified into the following two categories :
(i) Non-narcotic (non-addictive) drugs Example : Aspirin, Ibuprofen
(ii) Narcotic (addictive) drugs Example : Morphine, Heroin.
They are recommended with proper prescription because they are habit forming drugs.
Question 14.
Explain the following terms with one suitable example in each case.
(i) Cationic detergents
(ii) Enzymes
(iii) Antifertility drugs (Delhi 2010)
Answer:
(i) Cationic detergents : They are quaternary ammonium salts of amines with acetates, chlorides or bromides as anions and the cationic part possess a long hydrocarbon chain, and a positive charge on nitrogen atom. Therefore they are called cationic detergents.

(ii) Enzymes :
The enzymes may be defined as bio-catalysts which catalyse the bio-chemical reactions in the living organisms.
Example : Pepsin and Amylase.
(iii) Antifertility drugs : Chemical substances, which are used to check pregnancy in women, are called antifertility drugs or birth control pills or oral contraceptives. These control the female menstrual cycle and ovulation.
Example : Norethindrone, Ethinyl estradol, Mestranol.
Question 15.
Explain the following terms with one example in each case :
(i) Food preservatives
(ii) Enzymes
(iii) Detergents (Delhi 2010)
Answer:
(i) Food preservatives : They are used to prevent spoilage of food due to microbial growth.
Example : Table salt, vegetable oils, sodium benzoate etc.
(ii) Enzymes : The enzymes may be defined as bio-catalysts which catalyse the bio-chemical reactions in the living organisms.
Example : Pepsin and Amylase.
(iii) Detergents : They may be defined as ammonium, sulphonate or sulphate salts of long chain hydrocarbons containing 12-18 carbon atoms.
Example : Sodium lauryl sulphate.
Unlike soaps they are non-biodegradable and hence cause water pollution but they can be conveniently used even in hard water.
Question 16.
What are analgesic drugs? How are they classified and when are they usually recommended for use? (Delhi 2010)
Answer:
Analgesic drugs : These drugs are the chemical substances which are given to relieve body pains. These act on the central nervous system.
These are classified as Narcotics i.e. habit forming and non-narcotics i.e. not habit forming.
Examples of Narcotics : Opium which contains alkaloids such as Codeine and Morphine.
Examples of Non-Narcotics : Aspirin and Ibuprofen.
Question 17.
Explain the following terms with an example for each :
(i) Antibiotics
(ii) Antiseptics
(iii) Analgesics (Delhi 2010)
Answer:
(i) Antibiotics : Those chemical substances which are produced completely or partially by chemical synthesis in low concentration and either kill or inhibit the growth of microorganisms by intervening in their metabolic processes, are known as antibiotics.
Examples:
Tetracycline ➝ Bacteriostatic antibiotics, Penicillin Bactericidal antibiotics
(ii) Antiseptics :
These are chemical substances which prevent the growth of micro-organisms and may even kill them and safe to be applied on living tissues.
Example : Furacin, soframycin etc.
(iii) Analgesics :
Analgesic drugs : These drugs are the chemical substances which are given to relieve body pains. These act on the central nervous system.
These are classified as Narcotics i.e. habit forming and non-narcotics i.e. not habit forming.
Examples of Narcotics : Opium which contains alkaloids such as Codeine and Morphine.
Examples of Non-Narcotics : Aspirin and Ibuprofen.
Question 18.
Describe the following giving one example for each :
(i) Detergents
(ii) Food preservatives
(iii) Antacids (Delhi 2010)
Answer:
(i) Detergents :
They may be defined as ammonium, sulphonate or sulphate salts of long chain hydrocarbons containing 12-18 carbon atoms.
Example : Sodium lauryl sulphate.
Unlike soaps they are non-biodegradable and hence cause water pollution but they can be conveniently used even in hard water.
(ii) Food preservatives: They are used to prevent spoilage of food due to microbial growth.
Example : Table salt, vegetable oils, sodium benzoate etc.
(iii) Antacids : The substances which neutralize the excess acid and raise the pH to an appropriate level in the stomach are called antacids.
Example: Sodium bicarbonate, Ranitidine etc.
Question 19.
Explain the following terms with one suitable example for each :
(i) A sweetening agent for diabetic patients
(ii) Enzymes
(iii) Analgesics (Delhi 2010)
Answer:
(i) The sweetening agent used in the preparation of sweets for a diabetic patient is Saccharin.
(ii) Enzymes :
The enzymes may be defined as bio-catalysts which catalyse the bio-chemical reactions in the living organisms.
Example : Pepsin and Amylase.
(iii) Analgesics :
Analgesic drugs : These drugs are the chemical substances which are given to relieve body pains. These act on the central nervous system.
These are classified as Narcotics i.e. habit forming and non-narcotics i.e. not habit forming.
Examples of Narcotics : Opium which contains alkaloids such as Codeine and Morphine.
Examples of Non-Narcotics : Aspirin and Ibuprofen.
Question 20.
Answer the following questions :
(i) Why do soaps not work in hard water?
(ii) What are the main constituents of dettol?
(iii) How do antiseptics differ from disinfectants? (Delhi 2010)
Answer:
(i) Hard water contains insoluble calcium and magnesium chlorides which forms insoluble precipitate (scum) with soap and thus cannot be rinsed off easily.
(ii) Dettol is mixture of chloroxylenol and α-terpineol in a suitable solvent.
(iii)
| Antiseptics | Disinfectants |
| 1. Antiseptics either kill or prevent the growth of microorganisms. | 1. Disinfectants kill the microbes definitely. |
| 2. Antiseptics do not cause harm to the living tissues. | 2. Disinfectants are toxic to the living tissues and thus cause harm to the tissues of the skin etc. |
Question 21.
What are the following substances? Give one example of each.
(i) Food preservatives
(ii) Synthetic detergents
(iii) Antacids (All India 2010)
Answer:
(i) Food preservatives : They are used to prevent spoilage of food due to microbial growth.
Example : Table salt, vegetable oils, sodium benzoate etc.
(ii) Synthetic detergents : Synthetic detergents are cleansing agents which have all the properties of soap but which actually do not contain any soap.
Example : Sodium lauryl sulphate (or any one other)
(iii) Antacids :
The substances which neutralize the excess acid and raise the pH to an appropriate level in the stomach are called antacids.
Example: Sodium bicarbonate, Ranitidine etc.
Question 22.
(a) Differentiate between a disinfectant and an antiseptic. Give one example of each.
(b) What is tincture of iodine and what is it used for? (All India 2010)
Answer:
(a)
| Antiseptics | Disinfectants |
| 1. They are chemical substances which prevent the growth of micro-organisms and may even kill them. | 1. They are chemical substances which kill micro-organisms. |
| 2. They are safe to be applied to the living tissues. | 2. They are not safe to be applied to the living tissues. |
| 3. They are generally applied on wounds, cuts, ulcers and diseased skin surfaces. Example : Furacin, soframycin, dettol and savlon, 0.2% solution of phenol. | 3. They are used to kill micro-organisms present in the drains, toilets, floors etc. Example: Phenol (> 1% solution) and chlorine (0.2 to 0.4 ppm). |
(b) Tincture of iodine is 2-3% solution of iodine in alcohol and water.
- Use : It is used as a powerful antiseptic and applied on wounds to kill and prevent growth of micro-organisms.
Question 23.
What are the following substances? Give one example of each one of them. (Delhi 2012)
(i) Tranquilizers
(ii) Food preservatives
(iii) Synthetic detergents
Answer:
(i) Tranquilizers : Tranquilizers are chemical compounds used for the treatment of stress and mild or even severe mental diseases. Example : Equanil, meprobamate, veronal. (any one)
(ii) Food preservatives : Food preservatives are the compounds which prevent spoilage of food due to microbial growth.
Example : Sodium benzoate, table salt, vegetable oils etc.
(iii) Synthetic detergents : Synthetic detergents are cleansing agents which have all the properties of soap but which actually do not contain any soap.
Example : Sodium lauryl sulphate.
Question 24.
Explain the following terms giving one example of each type : (Delhi 2012)
(i) Antacids
(ii) Disinfectants
(iii) Enzymes
Answer:
(i) Antacids: Those substances which neutralize the excess acid and raise the pH to an appropriate level in stomach are called antacids.
Example : Sodium bicarbonate, Mg(OH)2
(ii) Disinfectants : Disinfectants are chemical compounds which kill microorganisms but are not safe when applied on living organisms.
Example : Phenol, chlorine.
(iii) Enzymes : Enzymes are biological catalysts which are chemically globular proteins having high molecular mass and highly specific in their actions due to presence of active sites of definite shape and size on their surfaces so that only specific substrate can fit in them. Example : Pepsin, amylase
Question 25.
What are the following substances ? Give one example of each.
(i) Antihistamines
(ii) Tranquilizers
(iii) Broad spectrum antibiotics (Comptt. Delhi 2012)
Answer:
(i) Antihistamines : Antihistamines are amines which are used as drugs to control the allergy effects produced by histamines. Example : Terfenadine
(ii) Tranquilizers : Tranquilizers are a class of chemical compounds used for the treatment of stress, and mild or even severe mental disease.
Example : Equanil.
(iii) Broad spectrum antibiotics : Antibiotics which kill or inhibit a wide range of Gram¬positive and Gram-negative bacteria are said to be broad spectrum antibiotics.
Example : Chloroamphenicol.
Question 26.
(a) How do antiseptics differ from disinfectants? Give one example of each. (Give two differences)
(b) Why do soaps not work in hard water? (Comptt. Delhi 2012)
Answer:
(a)
| Antiseptics | Disinfectants |
| 1. They are chemical substances which prevent the growth of micro-organisms and may even kill them. | 1. They are chemical substances which kill micro-organisms. |
| 2. They are safe to be applied to the living tissues. | 2. They are not safe to be applied to the living tissues. |
| 3. They are generally applied on wounds, cuts, ulcers and diseased skin surfaces. Example : Furacin, soframycin, dettol and savlon, 0.2% solution of phenol. | 3. They are used to kill micro-organisms present in the drains, toilets, floors etc. Example: Phenol (> 1% solution) and chlorine (0.2 to 0.4 ppm). |
(b) Hard water contains Ca+2 and Mg+2 ions. These ions form insoluble calcium and magnesium salts respectively, when Na or K soaps are dissolved in hard water. These insoluble salt, separate as scum which adheres to fabric, thereby making soap ineffective for cleansing action.
Question 27.
What are the following substances ? Give one example of each.
(i) Analgesics
(ii) Antibiotics
(iii) Tranquilizers (Comptt. All India 2012)
Answer:
(i) Analgesics : Analgesics reduce or abolish pain without causing impairment of consciousness, mental confusion, incoordination or paralysis or some other disturbances of nervous system.
Example : Aspirin.
(ii) Antibiotics : Antibiotic refers to a substance produced wholly or partly by chemical synthesis which in low concentration inhibits the growth or destroys microorganisms by intervening in their metabolic processes.
Example : Penicillin.
(iii) Tranquilizers : Tranquilizers are a class of chemical compounds used for the treatment of stress, and mild or even severe mental disease.
Example : Equanil.
Question 28.
What are the following substances? Give one example of each.
(i) Broad Spectrum antibiotics
(ii) Narcotic analgesics
(iii) Synthetic detergents (Comptt. All India 2012)
Answer:
(i) Broad spectrum antibiotics : Antibiotics which kill or inhibit a wide range of Gram¬positive and Gram-negative bacteria are said to be broad spectrum antibiotics.
Example : Chloroamphenicol.
(ii) Narcotic analgesics : Narcotic analgesics are administered in medicinal doses, relieve pain and produce sleep,
Example : Morphine and many of its homologues.
(iii) Synthetic detergents : Synthetic detergents are cleansing agents which have all the properties of soap but which actually do not contain any soap.
Example : Sodium lauryl sulphate.
Question 29.
(a) Which one of the following is a food preservative?
Equanil, Morphine, Sodium benzoate
(b) Why is bithional added to soap?
(c) Which class of drugs is used in sleeping pills? (Delhi 2013)
Answer:
(a) Sodium Benzoate: It is a food preservative.
(b) Bithional acts as deodorant in soaps, hence it works as an antiseptic agent and reduces the odours produced by bacterial decomposition of organic matter on the skin.
(c) Tranquilizers like barbiturates are used in sleeping pills.
Question 30.
(i) What class of drug is Ranitidine?
(ii) If water contains dissolved Ca2+ ions, out of soaps and synthetic detergents, which will you use for cleaning clothes?
(iii) Which of the following is an antiseptic? 0.2% phenol, 1% phenol (All India 2013)
Answer:
(i) Ranitidine is an Antacid.
(ii) We will use synthetic detergents because they can produce lather with the hard water containing Ca2+ ions.
(iii) 0.2% phenol acts as an antiseptic.
Question 31.
(a) How do antiseptics differ from disinfectants? Give one example of each.
(b) What are tranquilizers? Give one example. (Comptt. All India 2013)
Answer:
(a) AntisepticsDisinfectants1. They are chemical substances which prevent the growth of micro-organisms and may even kill them.1. They are chemical substances which kill micro-organisms.2. They are safe to be applied to the living tissues.2. They are not safe to be applied to the living tissues.3. They are generally applied on wounds, cuts, ulcers and diseased skin surfaces. Example : Furacin, soframycin, dettol and savlon, 0.2% solution of phenol.3. They are used to kill micro-organisms present in the drains, toilets, floors etc.
Example: Phenol (> 1% solution) and chlorine (0.2 to 0.4 ppm).
(b) Tranquilizers : Drugs which are used for the treatment of stress, fatigues, mild and severe mental diseases are called tranquilizers.
Example : Iproniazid, Phenelzine etc.
Question 32.
Explain the following and give one example for each :
(i) Broad spectrum antibiotics
(ii) Antipyretics
(iii) Anti-oxidants (Comptt. All India 2013)
Answer:
(i) Broad spectrum antibiotics : Antibiotics which kill or inhibit a wide range of Gram¬positive and Gram-negative bacteria are called broad spectrum antibiotics.
Example : Chloro-amphical
(ii) Antipyretics : Chemicals, which are used to bring down the body temperature during high fever, are called antipyretics Example : Paracetamol, Aspirin etc.
(iii) Anti-oxidants : Those molecules, which inhibit the oxidation of other molecules, are called anti-oxidants
Example : Thiols, Ascorbic acid etc.
Question 33.
(i) Give two examples of macromolecules that are chosen as drug targets.
(ii) What are antiseptics? Give an example.
(iii) Why is use of aspartame limited to cold foods and soft drinks? (Delhi 2014)
Answer:
(i) Carbohydrates and proteins
(ii) Antiseptics : These are chemical substances which prevent the growth of micro-organisms and may even kill them and safe to be applied on living tissues.
Example : Furacin, soframycin etc.
(iii) Because it decomposes at baking or cooking temperature.
Question 34.
(i) Name the sweetening agent used in the preparation of sweets for a diabetic patient.
(ii) What are antibiotics? Give an example.
(iii) Give two examples of macromolecules that are chosen as drug targets. (Delhi 2014)
Answer:
(i) Saccharin is used for a diabetic patient for preparation of sweets.
(ii) Antibiotics :
Those chemical substances which are produced completely or partially by chemical synthesis in low concentration and either kill or inhibit the growth of microorganisms by intervening in their metabolic processes, are known as antibiotics.
Examples:
Tetracycline ➝ Bacteriostatic antibiotics, Penicillin Bactericidal antibiotics
(iii) Carbohydrates, proteins, nucleic acid etc.
Question 35.
(i) What are disinfectants? Give an example.
(ii) Give two examples of macromolecules that are chosen as drug targets.
(iii) What are anionic detergents? Give an example. (Delhi 2014)
Answer:
(i) Disinfectants : Disinfectants are chemical compounds which kill microorganisms but are not safe when applied on living organisms.
Example : Phenol, chlorine.
(ii) Macromolecules used as drug targets are carbohydrates, proteins, nucleic acid and lipids.
(iii) Anionic detergents.
Those detergents in which large part of their molecules are anions and used in cleansing action, are called anionic detergents.
Example : Sodium alkyl sulphates These are obtained from long straight chain alcohols containing 12-18 carbon atoms by treatment with cone. H2SO4 followed by neutralization with NaOH.
Example : Sodium lauryl sulphate.

Question 36.
Explain the following terms with a suitable example for each:
(i) Disinfectants
(ii) Antacids
(iii) Food preservatives (Comptt. Delhi 2014)
Answer:
(i) Disinfectants : These are the chemical substances which are used for killing or
preventing the growth of micro-organisms but they are not safe for living tissues.
(ii) Antacid : Those substances which neutralize the excess acid and raise the pH to an appropriate level in stomach are called antacids.
Example : Sodium bicarbonate, Mg(OH)2
(iii) Food preservatives : They are used to prevent spoilage of food due to microbial growth.
Example : Table salt, vegetable oils, sodium benzoate etc.
Question 37.
What are the following? Give one example of each.
(i) Sweetening agents
(ii) Food preservatives
(iii) Antibiotics (Comptt. Delhi 2014)
Answer:
(i) Sweetening agent : Those chemical substances which are sweet in taste but do not add any calories to our body are called artificial sweetening agents. These are excreted as such through urine.
Example ; Saccharin, aspartame etc.
(ii) Food preservatives: They are used to prevent spoilage of food due to microbial growth.
Example : Table salt, vegetable oils, sodium benzoate etc.
(iii) Antibiotics : Those chemical substances which are produced completely or partially ’by chemical synthesis in low concentration and either kill or inhibit the growth of micro-organisms by intervening in their metabolic processes, are known as antibiotics.
Example : Tetracycline, Vancomycin
Question 38.
What are biodegradable and non-biodegradable detergents? Give one example of each. (Comptt. Delhi 2014)
Answer:
Biodegradable detergents : Detergents, having straight hydrocarbon chains are easily degraded or decomposed by micro-organism, are known as biodegradable detergents.
Example : Sodium lauryl sulphate.
Non-biodegradable detergents : Detergents containing branched hydrocarbon chains and are not easily decomposed by the micro-organisms, are known as non-biodegradable detergents.
Example : Sodium 4 – (1, 3, 5, 7-tetramethyloctyl) benzene sulphonate.
Question 39.
What is meant by the following terms? Explain with an example for each.
(i) Target molecules as used in medicinal chemistry
(ii) Food preservative
(iii) Non-ionic detergents (Comptt. All India 2014)
Answer:
(i) Drugs interact with macromolecules like proteins, carbohydrates, lipids etc. and are called as target molecules.
(ii) Food preservatives: They are used to prevent spoilage of food due to microbial growth.
Example : Table salt, vegetable oils, sodium benzoate etc.
(iii) Non-ionic detergents : These are esters of high molecular mass alcohols obtained by reaction between polyethylene glycol and steric acid.
Example :

Question 40.
Answer the following questions :
(i) Why should medicines not be taken without consulting a doctor?
(ii) What is meant by ‘broad spectrum antibiotics’?
(iii) What are the main constituents of Dettol? (Comptt. All India 2014)
Answer:
(i) Because medicines can cause harm to human body if a person does not know its physiological function on body.
(ii) Antibiotics which kill or inhibit a wide range of gram positive and gram negative bacteria, are called broad spectrum antibiotics.
(iii) Dettol is mixture of chloroxylenol and a-terpineol in a suitable solvent.
Question 41.
Answer the following :
(i) Why is the use of aspartame limited to cold foods and drinks?
(ii) How do antiseptics differ from disinfectants?
(iii) Why do soaps not work in hard water? (Comptt. All India 2014)
Answer:
(i) Use of aspartame is limited to cold foods and soft drinks because it is unstable at cooking temperature.
(ii)
| Antiseptics | Disinfectants |
| 1. They are chemical substances which prevent the growth of micro-organisms and may even kill them. | 1. They are chemical substances which kill micro-organisms. |
| 2. They are safe to be applied to the living tissues. | 2. They are not safe to be applied to the living tissues. |
| 3. They are generally applied on wounds, cuts, ulcers and diseased skin surfaces. Example : Furacin, soframycin, dettol and savlon, 0.2% solution of phenol. | 3. They are used to kill micro-organisms present in the drains, toilets, floors etc. Example: Phenol (> 1% solution) and chlorine (0.2 to 0.4 ppm). |
(iii) Hard water contains insoluble calcium and magnesium chlorides which forms insoluble ppt (scum) with soap and thus cannot be rinsed off easily.
Question 42.
Define the following:
(i) Anionic detergents
(ii) Broad spectrum antibiotics
(iii) Antiseptic (Delhi 2017)
Answer:
(i) Anionic detergents : Those detergents in which large part of their molecules are anions and used in cleansing action, are called anionic detergents.
Example : Sodium alkyl sulphates These are obtained from long straight chain alcohols containing 12-18 carbon atoms by treatment with cone. H2S04 followed by neutralization with NaOH.
Example : Sodium lauryl sulphate.

(ii) Broad spectrum antibiotics : Antibiotics which kill or inhibit a wide range of Gram¬positive and Gram-negative bacteria are said to be broad spectrum antibiotics.
Example : Chloroamphenicol.
(iii) Antiseptics are the chemicals which either kill or prevent growth of microbes on living tissues.
Question 43.
Define the following:
(i) Cationic detergents
(ii) Narrow spectrum antibiotics
(iii) Disinfectants (Delhi 2017)
Answer:
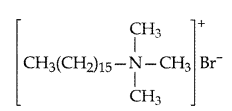
(i) Cationic detergents. They are quaternary ammonium salts of amines with acetates, chlorides or bromides as anions and the cationic part possess a long hydrocarbon chain, and a positive charge on nitrogen atom. Therefore they are called cationic detergents.
Example: Cetyltrimethyl ammonium bromide —
(ii) Narrow spectrum antibiotics. Narrow spectrum antibiotics are those antibiotics which are mainly effective against gram positive or gram negative bacteria.
(iii) Disinfectants. Disinfectants kill or prevent growth of microbes and are applied on inanimate/non living objects.
Example: Phenol
Question 44.
Define the following:
(i) Anionic detergents
(ii) Limited spectrum antibiotics
(iii) Tranquilizers (Delhi 2017)
Answer:
(i) Those detergents in which large part of their molecules are anions and used in cleansing action, are called anionic detergents.
Example: Sodium alkyl sulphates
(ii) Limited spectrum antibiotics are those which are effective against a single organism or disease.
(iii) Tranquilizers are class of chemicals used for treatment of stress or mild or severe mental diseases.
Question 45.
Define the following
(a) Anionic detergents
(b) Limited spectrum antibiotics
(c) Antiseptics (All India 2017)
Answer:
(a) Anionic detergents : Those detergents in which large part of their molecules are anions and used in cleansing action, are called anionic detergents.
Example : Sodium alkyl sulphates These are obtained from long straight chain alcohols containing 12-18 carbon atoms by treatment with cone. H2S04 followed by neutralization with NaOH.
Example : Sodium lauryl sulphate.

(b) Limited spectrum antibiotics are effective against a single organism or disease, e.g., Streptomycin.
(c) Antiseptics are the chemicals which either kill or prevent growth of microbes on living tissues, e.g., Penicillin.
Question 46.
Define the following:
(a) Anionic detergents
(b) Narrow spectrum antibiotics
(c) Antacids (All India 2017)
Answer:
(a) Anionic detergents : Those detergents in which large part of their molecules are anions and used in cleansing action, are called anionic detergents.
Example : Sodium alkyl sulphates These are obtained from long straight chain alcohols containing 12-18 carbon atoms by treatment with cone. H2S04 followed by neutralization with NaOH.
Example : Sodium lauryl sulphate.

(b) Narrow spectrum antibiotics are those which are effective against either gram positive or gram negative bacteria.
(c) Antacids are chemical compounds which are used for the treatment of excess acid produced in the stomach.
Question 47.
Define the following:
(a) Cationic detergents
(b) Broad spectrum antibiotics
(c) Tranquilizers (All India 2017)
Answer:
(a)
(i) Cationic detergents. They are quaternary
ammonium salts of amines with acetates, chlorides or bromides as anions and the cationic part possess a long hydrocarbon chain, and a positive charge on nitrogen atom. Therefore they are called cationic detergents.
Example: Cetyltrimethyl ammonium bromide —

(ii) Narrow spectrum antibiotics. Narrow spectrum antibiotics are those antibiotics which are mainly effective against gram positive or gram negative bacteria.
(iii) Disinfectants. Disinfectants kill or prevent growth of microbes and are applied on inanimate/non living objects.
Example: Phenol
(b) Broad spectrum antibiotics. Antibiotics which kill or inhibit a wide range of gram-positive and gram-negative bacteria.
(c) Tranquilizers. Chemical compounds used for the treatment of stress and mild or severe mental diseases.
Question 48.
Write the therapeutic action of following on human body and mention the class of drugs to which each of these belong:
(i) Ranitidine
(ii) Morphine
(iii) Aspirin (Comptt. Delhi 2017)
Answer:
(i) Ranitidine belongs to antacids and it neutralizes the excess acid and raises the pH to an appropriate level in stomach.
(ii) Morphine belongs to narcotic analgesics and it relieves pain and produces sleep even when taken in small dose.
(iii) Aspirin belongs to non-narcotic analgesics and it inhibits the synthesis of compounds which stimulate inflammation in the tissues and cause pain. Aspirin relieves pain and reduces fever.
Question 49.
Write the therapeutic action of following on human body and mention the class of drugs to which each of the these belong:
(i) Equanil
(ii) Aspirin
(iii) Chloramphenicol (Comptt. Delhi 2017)
Answer:
(i) Equanil belongs to the class of tranquilizers and it is used in controlling depression and hypertension.
(ii) Aspirin belongs to non-narcotic analgesics and it inhibits the synthesis of compounds which stimulate inflammation in the tissues and cause pain. Aspirin reduces pain and fever.
(iii) Chloramphenicol belongs to antibiotics and it is used for treatment of typhoid. It kills or inhibits the growth of micro-organisms.
Question 50.
(i) Name a substance which can be used as an antiseptic as well as disinfectant.
(ii) Name an artificial sweetener whose use is limited to cold foods and drinks.
(iii) What are cationic detergents? (Comptt. All India 2017)
Answer:
(i) Phenol
(ii) Aspartame
(iii) Cationic detergents. They are quaternary ammonium salts of amines with acetates, chlorides or bromides as anions and the cationic part possess a long hydrocarbon chain, and a positive charge on nitrogen atom. Therefore they are called cationic detergents.
Example: Cetyltrimethyl ammonium bromide —
