Important Questions for Class 12 Chemistry Chapter 14 Biomolecules Class 12 Important Questions
Biomolecules Class 12 Important Questions Very Short Answer Type
Question 1.
What is meant by ‘reducing sugars’? (All India 2010)
Answer:
Reducing sugar contains aldehydic or ketonic group in the hemiacetal and hemiketal forms and can reduce
Tollen’s reagent or Fehlmg’s solution.
Question 2.
What are monosaccharides? (All India 2010)
Answer:
These are the simplest carbohydrates which cannot be hydrolysed to smaller molecules. Their general formula is (CH2O)n where n = 3 – 7
Example : glucose, fructose etc.
Question 3.
Write the structure of the product obtained when glucose is oxidised with nitric acid. (All India 2012)
Answer:
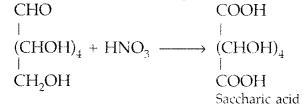
Question 4.
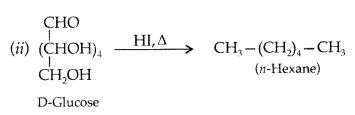
Write a reaction which shows that all the carbon atoms in glucose are linked in a straight chain. (All India 2012)
Answer:
On prolonged heating with HI, it forms n-hexane, shows that all the six carbon atoms are linked in a straight
chain :
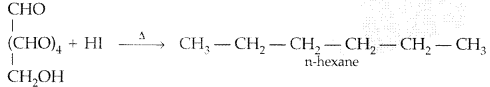
Question 5.
What are the expected products of hydrolysis of lactose ? (Comptt. Delhi 2012)
Answer:
On hydrolysis, lactose gives P-D-galactose and p-D-glucose.
Question 6.
Where does the water present in the egg go after boiling the egg? (Comptt. Delhi 2012)
Answer:
Denaturation of proteins is a process that changes the physical and biological properties of proteins without affecting the chemical composition of protein. In an egg, denaturation of protein is the coagulation of albumin present in the white of an egg. When an egg is boiled in water, the globular proteins present in it change to a rubber like insoluble mass which absorbs all the water present in the egg by making hydrogen bond with it.
Question 7.
Name a water soluble vitamin which is a powerful antioxidant. Give its one natural source. (Comptt. Delhi 2012)
Answer:
Water soluble vitamin : Vitamin C
Natural source : Amla
Question 8.
What are three types of RNA molecules which perform different functions? (Delhi 2013)
Answer:
m-RNA, t-RNA, r-RNA
Question 9.
What is a glycosidic linkage? (Delhi 2013)
Answer:
The two monosaccharide units are joined together through an etheral or oxide linkage formed by loss of a molecule of water. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage.
Question 10.
What are the products of hydrolysis of sucrose? (All India 2013)
Answer:
Invert sugar: An equimolar mixture of glucose and fructose is obtained by hydrolysis of sucrose in presence of an acid such as dil. HC1 or the enzyme invertase or sucrase and is called invert sugar.
Question 11.
Write the name of linkage joining two amino acids. (All India 2013)
Answer:
Peptide linkage joins two amino acids.
Question 12.
Name the deficiency diseases resulting from lack of Vitamins A and E in the diet. (Comptt. Delhi 2013)
Answer:
Deficiency of Vitamin A causes Xerophthalmia and deficiency of Vitamin E causes Sterility.
Question 13.
Name one water soluble vitamin which is a powerful antioxidant. Give its one natural source.
(Comptt. Delhi 2013)
Answer:
Water soluble vitamin : Vitamin C
Natural source : Amla
Question 14.
Name one oil soluble vitamin which is a powerful antioxidant and give its one natural source.
(Comptt. Delhi 2013)
Answer:
Oil soluble Vitamine : Vitamin D
Natural source : Fish liver oil, butter, milk, eggs etc.
Question 15.
Name the products of hydrolysis of lactose. (Comptt. All India 2013)
Answer:
Lactose on hydrolysis with dilute acids gives an equimolar mixture of D-glucose and D-galactose.
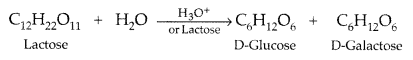
Question 16.
Name the only vitamin which can be synthesized in our body. Name the disease caused due to the
deficiency of this vitamin. (Comptt. All India 2013)
Answer:
Vitamin which can be synthesized in our body : Vitamin A
Its deficiency causes Xerophthalmia.
Question 17.
Mention one important function of nucleic acids in our body. (Comptt. All India 2013)
Answer:
Function of nucleic acid : Nucleic acids control the transmission of hereditary characters from one generation to another.
Question 18.
Which of the two components of starch is water soluble? (Delhi 2014)
Answer:
Amylose is water soluble component of starch.
Question 19.
Name the products of hydrolysis of sucrose. (Delhi 2014)
Answer:
Glucose and fructose are the products of hydrolysis of sucrose.
Question 20.
Which component of starch is a branched polymer of a-glucose and insoluble in water? (Delhi 2014)
Answer:
Amylopectin.
Question 21.
What are the products of hydrolysis of sucrose? (All India 2014)
Answer:
Glucose and fructose.
Question 22.
What are the products of hydrolysis of maltose? (All India 2014)
Answer:
![]()
Question 23.
Write the products of hydrolysis of lactose. (All India 2014)
Answer:
Lactose on hydroloysis with dilute acids gives an equimolar mixture of D-glucose and D-galactose.
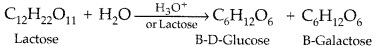
Question 24.
Define a ‘Peptide linkage’. (Comptt. All India 2014)
Answer:
Peptide linkage : It is an amide linkage formed between – COOH group of one α-amino acid and NH2 group of the other α-amino acid by loss of a molecule of water. – CO – NH – bond is called Peptide linkage.
Question 25.
What are enzymes? (Comptt. All India 2014)
Answer:
Enzymes are protein molecules which act as catalyst in biochemical reaction.
Biomolecules Class 12 Important Questions Short Answer Type SA-I
Question 26.
Explain what is meant by (Delhi 2009)
(i) a peptide linkage
(ii) a glycosidic linkage.
Answer:
(i) Peptide linkage: A peptide linkage is an amide linkage formed between – COOH group of one α-amino acid and NH2 group of the other a-amino acid by loss of a molecule of water.
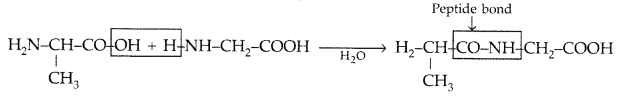
(ii) Glycosidic linkage : The two monosaccharide units are joined together through an etheral or oxide linkage formed by loss of a molecule of water. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage.
Question 27.
Name two water soluble vitamins, their sources and the diseases caused due to their deficiency in diet. (Delhi 2009)
Answer:
| Vitamins | Sources | Deficiency disease |
| 1. Vitamic B2
(Riboflavin or Lactoflavin) |
Milk, yeast, green vegetables, meat, liver, kidney, egg white etc. Daily dosage is 2-3 mg. | Retards growth, causes inflamation of tongue (glossitis), dermatitis and cheilosis (cracking or fissuring) at comers of mouth and lips. |
| 2. Vitamic C (Ascorbic acid) | Citrus fruits, green leafy vegetables, chillies, sprouted pulses and germinated grains. Daily dosage is 75 mg. | Scurvy (bleeding) of gums), pyorrhea (loosening and bleeding of teeth). |
Question 28.
Name the four bases present in DNA. Which one of these is not present in RNA? (All India 2009)
Answer:
The four bases present in DNA are :
(i) Adenine (A)
(ii) Guanine (G)
(iii) Cytosine (C)
(iv) Thymine (T)
In RNA, Thymine (T) is absent. It has Uracil (U) in place of Thymine.
Question 29.
Name two fat soluble vitamins, their sources and the diseases caused due to their deficiency in diet. (All India 2009)
Answer:
| Vitamin | Source | Deficiency disease |
|
1. Vitamin A |
Milk, butter, eggs, fish, liver oil, rice, kidney, green vegetables etc. | Xerophthalmia(hardening of cornea), night blindness and xerosis (drying of skin). |
| 2. Vitamin D | Fish liver oil, butter, milk, eggs, liver and meat. | Rickets, osteomalacia (soft bones and joint pain). |
Question 30.
Explain the following terms :
(i) Invert sugar
(ii) Polypeptides (Delhi 2009)
Answer:
(i) Invert sugar : An equimolar mixture of glucose and fructose obtained by hydrolysis of sucrose in presence of an acid such as dil. HCl or the enzyme invertase or sucrase is called invert sugar.
(ii) Polypeptides : They are formed when several molecules of a-amino acids are joined together by peptide bonds.
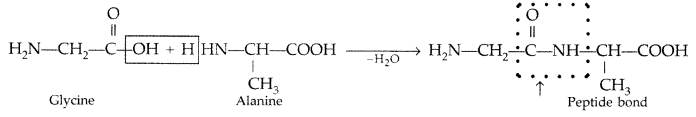
Question 31.
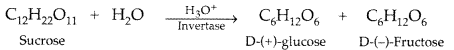
Name the products of hydrolysis of sucrose. Why is sucrose not a reducing sugar? (Delhi, All India 2009)
Answer:
Question 32.
What are essential and non-essential amino acids in human food? Give one example of each type.(Delhi 2009)
Answer:
Essential amino acids : Amino acids which the body cannot synthesize are called essential amino acids.
Example : Valine, leucine etc. Therefore they must be supplied in diet.
Non-essential amino acids : Amino acids which the body can synthesize are called non-essential amino acids. Therefore, they may or may not be present in diet.
Example : Glycine, alanine etc.
Question 33.
State clearly what are known as nucleosides and nucleotides. (Delhi 2009)
Answer:
Nucleoside : A nucleoside contains only two basic components of nucleic acids i.e. a pentose sugar and a nitrogenous base. During their formation 1-position of the pyrimidine or 9-position of the purine moitey is linked to C1 of the sugar (ribose or deoxyribose) by a β-linkage.
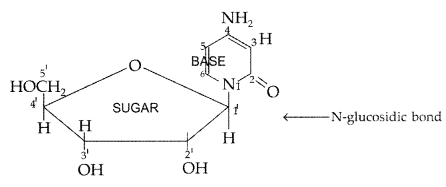
Nucleotides : A nucleotide contains all the three basic components of nucleic acids, i.e. a phosphoric acid group, a pentose sugar and a nitrogenous base. These are formed by esterification of C5‘ – OH of the sugar of the nucleoside with phosphoric acid.
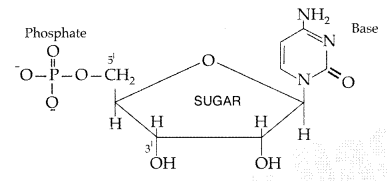
Question 34.
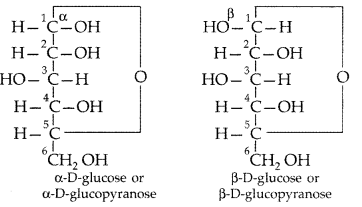
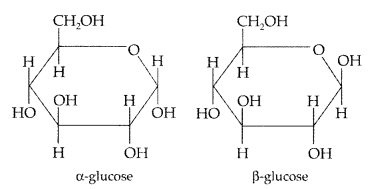
What is essentially the difference between a-form of glucose and p-form of glucose? Explain. (Delhi 2009)
Answer:
In a-α-glucose, the OH group at C1 is towards right while in p-D-glucose, the OH group at C1 is towards left.
Question 35.
Describe what you understand by primary structure and secondary structure of proteins. (Delhi 2009)
Answer:
Primary structure of proteins : Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence which is known as primary structure of protein.
Secondary structure of proteins : The conformation which the polypeptide chains assume as a result of hydrogen bonding is called the secondary structure of the protein.
Depending upon the size of the R groups, the two different secondary structures are possible which are :
- α-Helix structure : Intramolecular H-bonds present between the C = O of one amino acid and N – H of fourth amino acid.
- β-Pleated sheet structure : The two neighbouring polypeptide chains are held together by intermolecular H-bonds.
Question 36.
Explain what is meant by
(i) a peptide linkage,
(ii) a glycosidic linkage. (Delhi 2009)
Answer:
(i) Peptide linkage: A peptide linkage is an amide linkage formed between – COOH group of one α-amino acid and NH2 group of the other a-amino acid by loss of a molecule of water.
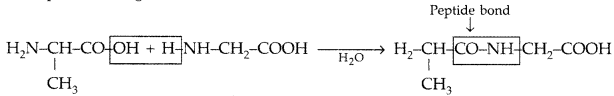
(ii) Glycosidic linkage : The two monosaccharide units are joined together through an etheral or oxide linkage formed by loss of a molecule of water. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage.
Question 37.
Name the bases present in RNA. Which one of these is not present in DNA? (Delhi 2009)
Answer:
The four bases present in RNA are :
Purines – Adenine (A) and Guanine (G)
Pyrimidines – Uracil (U) and Cytosine (C)
Uracil is not present in DNA.
Question 38.
Explain what is meant by the following :
(i) peptide linkage
(ii) pyranose structure of glucose (All India 2009)
Answer:
(i) Peptide linkage: A peptide linkage is an amide linkage formed between – COOH group of one a-amino acid and NH2 group of the other a-amino acid by loss of a molecule of water.
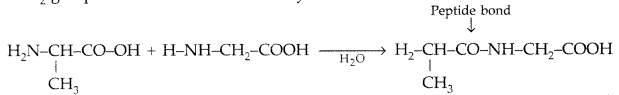
(ii) Pyranose structure of glucose : The six membered ring containing 5 carbon atoms and one oxygen atom because of its resemblance with pyron is called the pyranose form.
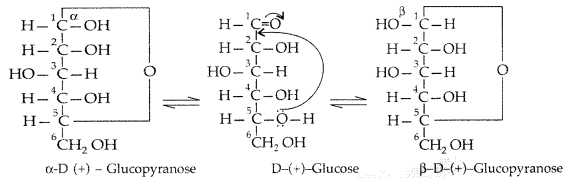
Question 39.
Write the main structural difference between DNA and RNA. Of the four bases, name those which are common to both DNA and RNA. (All India 2009)
Answer:
| DNA | RNA |
| 1. The sugar present in DNA is 2-deoxy-(-) ribose. | 1. The sugar present in RNA is D-(-) ribose. |
| 2. DNA contains cytosine and thymine as pyrimidine bases. | 2. RNA contains cytosine and uracil as pyrimidine bases. |
| 3. DNA has double standard α-helix structure. | 3. RNA has single stranded α-helix structure. |
The base which are common to both DNA and RNA are :
- Adenine (A)
- Guanine (G)
- Cytosine (C)
Question 40.
Write such reactions and facts about glucose which cannot be explained by its open chain structure. (All India 2009)
Answer:
Limitations of the open chain structure of glucose :
- Glucose does not form NaHSO3 addition product. Despite having aldehyde-ammonia group, it does not respond to 2,4-DNP test and does not respond to Schiff’s reagent test.
- Glucose penta acetate does not react with NH2OH due to absence of aldehydic group.
Question 41.
Write any two reactions of glucose which cannot be explained by the open chain structure of glucose molecule. (Delhi 2012)
Answer:
- Despite having the aldehyde group, glucose does not give 2, 4-DNP test or Schiff’s test.
- It does not form the hydrogen sulphite addition product with NaHSO3.
- The pentaacetate of glucose does not react with hydroxylamine indicating the absence of free – CHO group.
Question 42.
Write the main structural difference between DNA and RNA. Of the two bases, thymine and uracil, which one is present in DNA? (Delhi 2012)
Answer:
(i) Difference between DNA and RNA :
| DNA | RNA |
| 1. The sugar present in DNA is 2-deoxy-(-) ribose. | 1. The sugar present in RNA is D-(-) ribose. |
| 2. DNA contains cytosine and thymine as pyrimidine bases. | 2. RNA contains cytosine and uracil as pyrimidine bases. |
| 3. DNA has double standard α-helix structure. | 3. RNA has single stranded α-helix structure. |
The base which are common to both DNA and RNA are :
- Adenine (A)
- Guanine (G)
- Cytosine (C)
(ii) Thymine is present in DNA.
Question 43.
Write down the structures and names of the products formed when D-glucose is treated with
(i) Hydroxylamine
(ii) Acetic anhydride. (Comptt. All India 2012)
Answer:
(i) D-glucose reacts with hydroxylamine to form oxime.
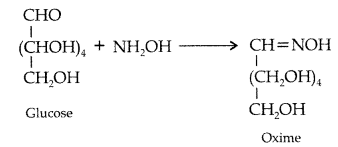
(ii) D-glucose reacts with acetic anhydride to give penta-acetate.
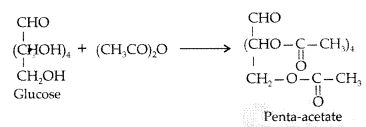
Question 44.
(a) Name the only vitamin which can be synthesized in our body. Name one disease that is caused due to the deficiency of this vitamin.
(b) State two functions of carbohydrates. (Comptt. All India 2012)
Answer:
(a) Vitamin that can be synthesized ‘.Vitamin B12
Disease due to the deficiency of Vitamin B12 : Pernicious anaemia.
(b) Two functions of glucose :
- Carbohydrates such as glucose, starch, glycogen etc. provide energy for functioning of living organisms.
- Carbohydrates, especially cellulose in the form of wood is used for making furniture, houses etc. by us.
Question 45.
Write down the structures and names of the products formed when D-glucose is treated with
(i) Bromine water
(ii) Hydrogen Iodide (Prolonged heating). (Comptt. All India 2012)
Answer:
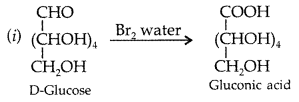
Question 46.
Answer the following questions:
(i) Why are vitamin A and vitamin C essential for us?
(ii) What is the difference between a nucleoside and a nucleotide? (Comptt. Delhi 2014)
Answer:
(i) Because deficiency of vitamin A and vitamin C causes night blindness and scurvy respectively.
(ii) Nucleoside : A nucleoside contains only two basic components of nucleic acids i.e. a pentose sugar and a nitrogenous base. During their formation 1-position of the pyrimidine or 9-position of the purine moitey is linked to C1 of the sugar (ribose or deoxyribose) by a β-linkage.

Nucleotides : A nucleotide contains all the three basic components of nucleic acids, i.e. a phosphoric acid group, a pentose sugar and a nitrogenous base. These are formed by esterification of C5‘ – OH of the sugar of the nucleoside with phosphoric acid.

Question 47.
Enumerate the reactions of glucose which cannot be explained by its open chain structures. (Comptt. Delhi 2014)
Answer:
Limitations of the open chain structure of glucose :
- Glucose does not form NaHS03 addition product. Despite having aldehyde-ammonia group, it does not give 2,4-DNP test and does not respond to Schiff’s reagent test.
- Glucose penta acetate does not react with NH2OH due to absence of aldehydic group.
Question 48.
Write the ambident nucleophiles? Give an example. (Comptt. Delhi 2014)
Answer:
A group containing two nucleophilic centres.
Example : –CN (Cyanide) and –NC (Isocynide).
Biomolecules Class 12 Important Questions Short Answer Type SA-II
Question 49.
Amino acids may be acidic, alkaline or neutral. How does this happen? What are essential and non-essential amino acids? Name one of each type. (All India 2010)
Answer:
Amino acids can be broadly classified into three classes i.e. acidic, alkaline and neutral amino acids depending on the number of —NH2 group and — COOH group.
Acidic amino acids : Those a-amino acids such as aspartic acid, asparagine and glutamic acid which contain two -COOH groups and one -NH2 group are called acidic amino acids.
Alkaline or Basic amino acids : Those a-amino acids such as lysine, arginine and histidine which
contain two -NH2 groups and one -COOH group, are called basic amino acids.
Neutral amino acids : Those a-amino acids such as glycine, alanine, valine etc. which contain one -NH2 and one – COOH group, are called neutral amino acids.
Essential amino acids : Amino acids which the body cannot synthesize are called essential amino acids.
Example : Valine, leucine etc. Therefore they must be supplied in diet.
Non-essential amino acids : Amino acids which the body can synthesize are called non-essential amino acids. Therefore, they may or may not be present in diet.
Example : Glycine, alanine etc.
Question 50.
Differentiate between fibrous proteins and globular proteins. What is meant by the denaturation of a protein? (All India 2010)
Answer:
|
Globular Proteins |
Fibrous Proteins |
| 1. Globular proteins have almost spheroidal shape due to folding of the polypeptide chain.
|
1. Polypeptide chains of fibrous proteins consist of thread like molecules which tend to lie side by side to form fibres.
|
| 2. Globular proteins are soluble in water. | 2. Fibrous proteins are insoluble in water. |
| 3. Globular proteins are sensitive to small changes of temperature and pH. Therefore they undergo denaturation on heating or on treatment with acids/bases | 3. Fibrous proteins are stable to moderate changes of temperature and pH. |
| 4. They possess biological activity that’s why they act as enzymes. | 4. They do not have any biological activity but serve as chief structural material of animal tissues. |
| Example: Maltase, invertase etc., hormones (insulin) antibodies, transport agents (haemoglobin), etc. | Example: Keratin in skin, hair, nails and wool, collagen in tendons, fibroin in silk etc. |
Denaturation of protein : Due to coagulation of globular protein under the influence of change in temperature, change in pH etc., the native shape of the protein is destroyed and biological activity is lost and the formed protein is called denaturated proteins and the phenomenon is denaturation.
Question 51.
What is essentially the difference between a-glucose and P-glucose? What is meant by pyranose structure of glucose? (All India 2012)
Answer:
The two cyclic hemiacetal forms of glucose differ only in the configuration of the hydroxyl group on the first carbon atom called anomeric carbon. Such isomers i.e, α-form and β-form are called anomers. a-glucose is the monomer unit of starch and P-glucose is the monomer unit of cellulose. The six membered cyclic structure of glucose is called pyranose structure.
Pyranose structure of glucose : The six membered ring containing 5 carbon atoms and one oxygen atom because of its resemblance with pyron is called the pyranose form.

Also study the following :
Question 52.
Define the following as related to proteins :
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation (All India 2012)
Answer:
(i) Peptide linkage : A peptide linkage is an amide linkage formed between – COOH group of one a-amino acid and NH2 group of the other a-amino acid by loss of a molecule of water. The-CO-NH-bond formed is called petide linkage.
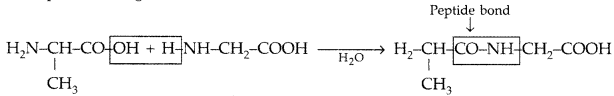
(ii) Primary structure : Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is called the primary structure of that protein.
(iii) Denaturation : Due to coagulation of globular protein under the influence of change in temperature, change in pH etc., the native shape of the protein is destroyed and biological activity is lost and the formed protein is called denaturated proteins and the phenomenon is denaturation.
Question 53.
What are the different types of RNA found in cells of organisms ? State the functions of each type. (Comptt. Delhi 2012)
Answer:
Different types of RNA found in the cell are :
- Messenger RNA (mRNA): carries the message of DNA for specific protein synthesis.
- Ribosotnal RNA (rRNA) : provides the site for protein synthesis.
- Transfer RNA (t-RNA): transfers amino acids to the site of protein synthesis.
Question 54.
(a) What are essential and non-essential amino acids? Give two examples of each type.
(b) What are the hydrolysis products of sucrose? (Comptt. Delhi 2012)
Answer:
(a) Non-essential amino acids : The amino acids which can be synthesised in the body, are known as non-essential amino acids. Example : Glycine, Alanine etc.
Essential amino acids : The amino acids which cannot be synthesised in the body and must be obtained through diet are known as essential amino acids.
Example : Valine, Leucine etc.
(b) Sucrose on hydrolysis gives equimolar mixture of D(+)-glucose and D(-) fructose
![]()
Question 55.
(a) Write the structural and functional differences between DNA and RNA
(b) Name two components of starch. (Comptt. Delhi 2012)
Answer:
(a) Structural difference :
| DNA | RNA |
| 1. The sugar present in DNA is 2-deoxy-(-) ribose. | 1. The sugar present in RNA is D-(-) ribose. |
| 2. DNA contains cytosine and thymine as pyrimidine bases. | 2. RNA contains cytosine and uracil as pyrimidine bases. |
| 3. DNA has double standard α-helix structure. | 3. RNA has single stranded α-helix structure. |
Adenine (A)The base which are common to both DNA and RNA are :
- Guanine (G)
- Cytosine (C)
Functional difference : DNA’s main function is to control cell activities like telling each organ what to make and what to do. RNA’s main function is to make protein.
(b) Components of starch : Amylose and amylopectin.
Question 56.
(a) Give two differences between globular and fibrous proteins.
(b) What change occurs in the nature of egg protein on boiling? (Comptt. Delhi 2013)
Answer:
(a)
|
Globular Proteins |
Fibrous Proteins |
| 1. Globular proteins have almost spheroidal shape due to folding of the polypeptide chain.
|
1. Polypeptide chains of fibrous proteins consist of thread like molecules which tend to lie side by side to form fibres.
|
| 2. Globular proteins are soluble in water. | 2. Fibrous proteins are insoluble in water. |
| 3. Globular proteins are sensitive to small changes of temperature and pH. Therefore they undergo denaturation on heating or on treatment with acids/bases | 3. Fibrous proteins are stable to moderate changes of temperature and pH. |
| 4. They possess biological activity that’s why they act as enzymes. | 4. They do not have any biological activity but serve as chief structural material of animal tissues. |
| Example: Maltase, invertase etc., hormones (insulin) antibodies, transport agents (haemoglobin), etc. | Example: Keratin in skin, hair, nails and wool, collagen in tendons, fibroin in silk etc. |
Denaturation of protein : Due to coagulation of globular protein under the influence of change in temperature, change in pH etc., the native shape of the protein is destroyed and biological activity is lost and the formed protein is called denaturated proteins and the phenomenon is denaturation.
(b) Because the egg comes in contact with a solution of higher osmotic pressure, the egg will shrink due to going out of water. This shrinking of egg is called plasmolysis.
Question 57.
(a) How are hormones and vitamins different in respect of their source and functions?
(b) Give one example each of
(i) Globular protein
(ii) Fibrous protein (Comptt. All India 2012)
Answer:
(a) Hormones are synthesized in our body and help in regulation of our body systems while vitamins are synthesized artificially in the laboratory or obtained from the food which helps in controlling many diseases.
(b)
- Globular protein : All enzymes and hormones like insulin.
- Fibrous protein : Keratin in skin, nails etc.
Question 58.
(a) What are essential and non-essential amino acids? Give two examples of each.
(b) How are nucleosides different from nucleotides? (Comptt. All India 2012)
Answer:
(a) Non-essential amino acids : The amino acids which can be synthesised in the body, are known as non-essential amino acids. Example : Glycine, Alanine etc.
Essential amino acids : The amino acids which cannot be synthesised in the body and must be obtained through diet are known as essential amino acids.
Example : Valine, Leucine etc.
(b)
Nucleoside : A nucleoside contains only two basic components of nucleic acids i.e. a pentose sugar and a nitrogenous base. During their formation 1-position of the pyrimidine or 9-position of the purine moitey is linked to C1 of the sugar (ribose or deoxyribose) by a β-linkage.

Nucleotides : A nucleotide contains all the three basic components of nucleic acids, i.e. a phosphoric acid group, a pentose sugar and a nitrogenous base. These are formed by esterification of C5‘ – OH of the sugar of the nucleoside with phosphoric acid.

Question 59.
(i) Deficiency of which vitamin causes night-blindness?
(ii) Name the base that is found in nucleotide of RNA only.
(iii) Glucose on reaction with HI gives n-hexane. What does it suggest about the structure of glucose? (Delhi 2014)
Answer:
(i) Vitamin A causes night blindness.
(ii) Uracil is found in nucleotide of RNA only.
(iii) It suggests the open structure of glucose.
Question 60.
(i) Deficiency of which vitamin causes rickets?
(ii) Give an example for each of fibrous protein and globular protein.
(iii) Write the product formed on reaction of D-glucose with Br2 water. (Delhi 2014)
Answer:
(i) Deficiency of Vitamin D causes rickets.
(ii) Fibrous protein ➝ α-keratin
Globular protein ➝ Insulin
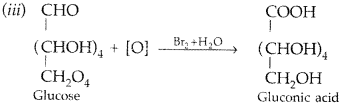
Question 61.
(i) Deficiency of which vitamin causes scurvy?
(ii) What type of linkage is responsible for the formation of proteins?
(iii) Write the product formed when glucose is treated with HI. (Delhi 2014)
Answer:
(i) Vitamin C causes scurvy.
(ii) Peptide linkages are responsible for the formation of proteins.
(iii) Glucose is treated with HI :
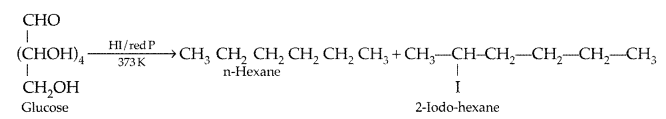
Question 62.
Define the following terms as related to proteins :
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation (All India 2014)
Answer:
(i) Peptide linkage : A peptide linkage is an amide linkage formed between – COOH group of one a-amino acid and NH2 group of the other a-amino acid by loss of a molecule of water. The-CO-NH-bond formed is called petide linkage.
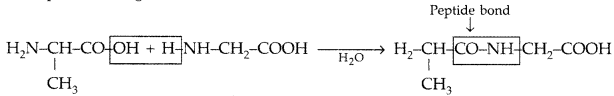
(ii) Primary structure : Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is called the primary structure of that protein.
(iii) Denaturation : Due to coagulation of globular protein under the influence of change in temperature, change in pH etc., the native shape of the protein is destroyed and biological activity is lost and the formed protein is called denaturated proteins and the phenomenon is denaturation.
Question 63.
Define the following terms :
(i) Glycosidic linkage
(ii) Invert sugar
(iii) Oligosaccharides (All India 2014)
Answer:
(i) Glycosidic linkage : The two monosaccharide units are joined together through an etheral or oxide
linkage formed by loss of a molecule of water. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage
(ii) Invert sugar : An equimolar mixture of glucose and fructose obtained by hydrolysis of sucrose in presence of an acid such as dil. HC1 or the enzyme invertase or sucrase is called invert sugar.
(iii) Oligosaccharides : Those carbohydrates which on hydrolysis give 2-10 molecules of monosaccharides are called oligosaccharides.
Example : sucrose, maltose.
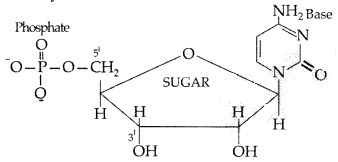
Question 64.
Define the following terms :
(i) Nucleotide
(ii) Anomers
(iii) Essential amino acids (All India 2014)
Answer:
(i) Nucleotide : A nucleotide contains all the three basic components of nucleic acids, i.e. a phosphoric acid group, a pentose sugar and a nitrogenous base. These are formed by esterification of C5 -OH of the sugar of the nucleoside with phosphoric acid.
Structure of Nucleotide:
(ii) Anomers : A pair of stereoisomers which differ in configuration only around C1 are called anomers. Two isomers are said to be anomers if the isomerisation in the molecule is at first carbon.
(iii) Essential amino acids: Essential amino acids : Amino acids which the body cannot synthesize are called essential amino acids. Example : Valine, leucine etc. Therefore they must be supplied in diet.
Non-essential amino acids : Amino acids which the body can synthesize are called non-essential amino acids. Therefore, they may or may not be present in diet.
Example ; Glycine, alanine etc.
Question 65.
What are essential and non-essential amino acids? Give two examples of each. (Comptt. All India 2014)
Answer:
Essential amino acids : Amino acids which the body cannot synthesize are called essential amino acids. Example : Valine, leucine etc. Therefore they must be supplied in diet.
Non-essential amino acids : Amino acids which the body can synthesize are called non-essential amino acids. Therefore, they may or may not be present in diet.
Example ; Glycine, alanine etc.
Question 66.
(i) Which one of the following is a disaccharide : Starch, Maltose, Fructose, Glucose?
(ii) What is the difference between fibrous protein and globular protein?
(iii) Write the name of vitamin whose deficiency causes bones deformities in children. (Delhi 2014)
Answer:
(i) Maltose is a disaccharide.
(ii)
|
Globular Proteins |
Fibrous Proteins |
| 1. Globular proteins have almost spheroidal shape due to folding of the polypeptide chain. | 1. Polypeptide chains of fibrous proteins consist of thread like molecules which tend to lie side by side to form fibres. |
| 2. Globular proteins are soluble in water. | 2. Fibrous proteins are insoluble in water. |
| 3. Globular proteins are sensitive to small changes of temperature and pH. Therefore they undergo denaturation on heating or on treatment with acids/bases | 3. Fibrous proteins are stable to moderate changes of temperature and pH. |
| 4. They possess biological activity that’s why they act as enzymes. | 4. They do not have any biological activity but serve as chief structural material of animal tissues. |
| Example: Maltase, invertase etc., hormones (insulin) antibodies, transport agents (haemoglobin), etc. | Example: Keratin in skin, hair, nails and wool, collagen in tendons, fibroin in silk etc. |
Denaturation of protein : Due to coagulation of globular protein under the influence of change in temperature, change in pH etc., the native shape of the protein is destroyed and biological activity is lost and the formed protein is called denaturated proteins and the phenomenon is denaturation.
(iii) Vitamin D
Question 67.
(i) Which one of the following is a polysaccharide :
Starch, Maltose, Fructose, Glucose?
(ii) What one difference between a-helix and P-pleated sheet structure of protein.
(iii) Write the name of the disease caused by the deficiency of Vitamin B12. (All India 2015)
Answer:
(i) Starch is a polysaccharide.
(ii) α-Helix structure : The polypeptide chains are held together (stabilized) by intramolecular H-bonding.
β-Pleated sheet structure : The two neighbouring polypeptide chains are held together by intermolecular , H-bonding.
(iii) Disease caused by the deficiency of Vitamin B12 is Pernicious anaemia.
Question 68.
How are vitamins classified? Name the vitamin responsible for the coagulation of blood. (Comptt. Delhi 2015)
Answer:
Vitamins are classified into two types :
- Water insoluble vitamins : These are fat soluble substances E.g. Vitamin A, D, E and K.
- Water soluble vitamins : These include Vitamin B-Complex and Vitamin C (except B12).
Vitamin K or phylloquinone is responsible for the coagulation of blood.
Question 69.
Define the following as related to proteins :
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation (Comptt. All India 2015)
Answer:
(i) Peptide linkage : A peptide linkage is an amide linkage formed between – COOH group of one a-amino acid and NH2 group of the other a-amino acid by loss of a molecule of water. The-CO-NH-bond formed is called peptide linkage.
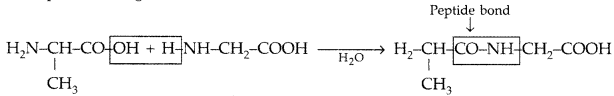
(ii) Primary structure : Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is called the primary structure of that protein.
(iii) Denaturation : Due to coagulation of globular protein under the influence of change in temperature, change in pH etc., the native shape of the protein is destroyed and biological activity is lost and the formed protein is called denaturated proteins and the phenomenon is denaturation.
Question 70.
(i) Write the name of two monosaccharides obtained on hydrolysis of lactose sugar.
(ii) Why Vitamin C cannot be stored in our body?
(iii) What is the difference between a nucleoside ’ and nucleotide? (Delhi 2016)
Answer:
(i) On hydrolysis, lactose gives β-D-ga lactose and β-D-glucose.
(ii) Vitamin C is mainly ascorbic acid which is water soluble and is readily excreted through urine and thus cannot be stored in the body.
(iii) Nucleoside. A nucleoside contains only two basic components of nucleic acids, i.e., a pentose sugar and a nitrogenous base. It is formed by the attachment of a base to V position of sugar.
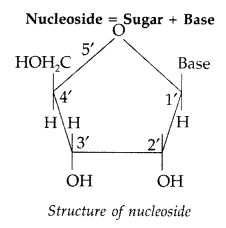
Nucleotides. A nucleotide contains all the three basic components of nucleic acids, i.e., a phosphoric acid group, a pentose sugar and nitrogenous base. These are formed by the esterification of C5—OH of the sugar of the nucleoside with phosphoric acid.
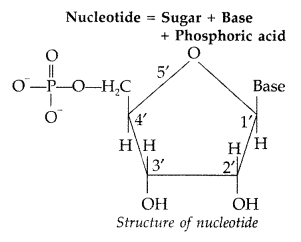
Question 71.
(i) Write the structural difference between starch and cellulose.
(ii) What type of linkage is present in Nucleic acids?
(iii) Give one example each for fibrous protein and globular protein. (All India 2016)
Answer:
(i) Starch contains the β-D-glucose as its monomer units while cellulose contains β-D- glucose as its monomer units.
(ii) Phosphodiester linkages are present in Nucleic Adds
(iii) Globular protein : All enzymes and hormones like insulin.
Fibrous protein : Keratin in skin.
Question 72.
Define the following as related to proteins :
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation (Comptt. Delhi 2016)
Answer:
(i) Peptide linkage : Two amino acids of same type or different types combine together by the elimination of H2O molecule to form – CONH- linkage.
(ii) Primary structure : It refers to the sequence in which amino acids are joined.
(iii) Denaturation : When a native protein is subjected to change in temperature or pH, hydrogen bonds get disturbed and globules get uncoiled and proteins lose their biological activity.
Question 73.
What are enzymes? Describe their functions. Name two diseases which are caused due to deficiency of enzymes. (Comptt. All India 2016)
Answer:
Enzymes are protein molecules which acts catalyst in Biochemical Reactions (biocatalyst). They increase the rate of biochemical reactions. For example : Zymase, Invertase etc.
Two diseases due to deficiency of enzymes are : Anemia, Gauchea’s disease.
Question 74.
(a) What type of linkage is present in disaccharides?
(b) Write one source and deficiency disease of vitamin B12.
(c) Write the difference between DNA and RNA. (Comptt. Delhi 2016)
Answer:
(a) Glycosidic linkage is present in disaccharides.
(b) Eggs are the source of Vitamin B12 and its deficiency causes pernicious anaemia.
(c) DNA is a double strand while RNA is a single strand molecule.
Question 75.
(a) What type of linkage is present in proteins?
(b) Give one example each of water soluble and fat soluble vitamins.
(c) Draw pyranose structure of glucose. (Comptt. Delhi 2016)
Answer:
(a) Peptide linkage is present in proteins.
(b) Vitamin C is water soluble and Vitamin D is fat soluble vitamin.
(c) Pyranose structure of glucose
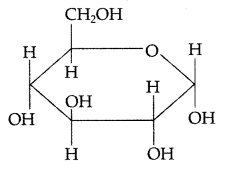
Question 76.
(a) Why water soluble vitamins must be supplied regularly in the diet? Give one example of it.
(b) Differentiate between the following :
(i) Essential and non-essential amino acids.
(ii) Fibrous and globular proteins.
Answer:
(a) Water soluble vitamins must be supplied regularly in the diet because they are regularly excreted in urine and cannot be stored in our body. For eg., Vitamin C, Vitamin B, etc.
(b)
(i) Essential amino acids : Amino acids which the body cannot synthesize are called essential amino acids. Example : Valine, leucine etc. Therefore they must be supplied in diet.
Non-essential amino acids : Amino acids which the body can synthesize are called non-essential amino acids. Therefore, they may or may not be present in diet.
Example ; Glycine, alanine etc.
(ii)
|
Globular Proteins |
Fibrous Proteins |
| 1. Globular proteins have almost spheroidal shape due to folding of the polypeptide chain.
|
1. Polypeptide chains of fibrous proteins consist of thread like molecules which tend to lie side by side to form fibres.
|
| 2. Globular proteins are soluble in water. | 2. Fibrous proteins are insoluble in water. |
| 3. Globular proteins are sensitive to small changes of temperature and pH. Therefore they undergo denaturation on heating or on treatment with acids/bases | 3. Fibrous proteins are stable to moderate changes of temperature and pH. |
| 4. They possess biological activity that’s why they act as enzymes. | 4. They do not have any biological activity but serve as chief structural material of animal tissues. |
| Example: Maltase, invertase etc., hormones (insulin) antibodies, transport agents (haemoglobin), etc. | Example: Keratin in skin, hair, nails and wool, collagen in tendons, fibroin in silk etc. |
Denaturation of protein : Due to coagulation of globular protein under the influence of change in temperature, change in pH etc., the native shape of the protein is destroyed and biological activity is lost and the formed protein is called denaturated proteins and the phenomenon is denaturation.