Acids, Bases and Salts Class 10 MCQs Questions with Answers
Question 1.
What happens when the solution of an acid is mixed the solution of a base in a test tube?
(I) The temperature of the solution increases
(II) The temperature of the solution decreases
(III) The temperature of the solution remains the same
(IV) Salt formation takes place
(a) Only (I)
(b) (I) and (III)
(c) (I) and (III)
(d) (I) and (IV)
Answer:
(d) (I) and (IV)
Explanation: When an acid reacts with a base, a neutral salt is formed by the neutralisation process. As the neutralisation process is an exothermic reaction, the temperature of the solution increases.
Acid + Base → Salt + Water
Question 2.
If 10 mL of H2SO4 is mixed with 10 mL of Mg(OH)2 of the same concentration, the resultant solution will give the following colour with a universal indicator:
(a) Red
(b) Yellow
(c) Green
(d) Blue
Answer:
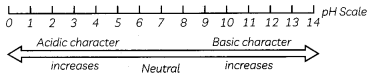
(c) Green.
Explanation: If 10 mL of H2SO4 is mixed with 10 mL of Mg(OH)2 of the same concentration, the resultant solution be MgS04 which is a neutral salt and universal indicator will give the green colour in this solution:
![]()
Related Theory:
When an acid reacts with a base it forms salt and water. As a result, acidic properties disappear The process is called neutralisation. For a neutral solution. pH is 7. The solution having pH 7 wilL turn green in colour in universal indicator.
Question 3.
Which of the following saLts does not contain water of crystatUsatlon?
(a) Blue vitriol
(b) Baking soda
(c) Washing soda
(d) Gypsum
Answer:
Question 4.
A visually challenged student, has to perform a lab test to detect the presence of acid in a given solution. The acid-base indicator preferred by him will be:
(a) Blue litmus
(b) Clove oil
(c) Red cabbage extract
(d) Hibiscus extract
Answer:
(b) Clove oil
Explanation: Clove oil is an olfactory indicator. These are the substances which give one type of odour in acidic medium so a visually challenged student prefers to use clove oil as an acid-base indicator.
As in basic solutions, the smell of clove oil disappears while the smell is retained when mixed with an acid, on the other hand, blue litmus, red cabbage extract and hibiscus extract wouLd not be used as acid-base indicator because in these indicators there will be a change in the colour.
Question 5.
Baking soda is a mixture of:
(a) Sodium carbonate and acetic acid
(b) Sodium carbonate and tartaric acid
(c) Sodium hydrogen carbonate and tartaric acid
(d) Sodium hydrogen carbonate and acetic acid
Answer:
Question 6.
Which of the following gives the correct increasing order of acid strength?
(a) Water < acetic acid < hydrochloric acid
(b) Water < hydrochloric acid < acetic acid
(c) Acetic acid < water < hydrochloric acid
(d) Hydrochloric acid < water < acetic acid
Answer:
Question 7.
What is observed when we pour a drop of acetic acid first on red and then on blue litmus papers?
(a) Red litmus paper becomes colourless and blue litmus paper remains blue.
(b) Red litmus paper turns blue and blue litmus paper remains blue.
(c) Red litmus paper remains red and blue litmus paper turns red.
(d) Red litmus paper turns blue and blue litmus paper turns red.
Answer:
Question 8.
Sodium hydrogen carbonate when added to acetic acid evolves a gas. Which of the following statements are true about the gas evolved?
(I) It turns lime water milky.
(II) It extinguishes a burning splinter.
(III) It dissolves in a solution of sodium hydroxide.
(IV) It has a pungent odour.
(a) (I) and (II)
(b) (I), (II) and (III)
(c) (II), (III) and (IV)
(d) (I) and (IV)
Answer:
(b) (I), (II) and (III)
Explanation: Reaction of sodium hydrogen carbonate with acetic acid forms sodium acetate and water with carbon dioxide (CO2) gas.
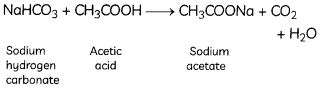
CO2 does not have a pungent smell but it shows all the other three properties:
- turns lime water milky,
- is a non-supporter of combustion and
- absorbed by strong alkalis such as NaOH.
Question 9.
Common salt besides being used in the kitchen can also be used as the raw material for making:
(I) Washing soda
(II) Bleaching powder
(III) Baking soda
(IV)Slaked Urne
(a) (I) and (II)
(b) (I), (II) and (IV)
(c) (I) and (III)
(d) (I), (III) and (IV)
Answer:
Question 10.
To protect tooth decay we are advised to brush our teeth regularly. The nature of the toothpaste commonly used ¡s:
(a) Acidic
(b) Neutral
(c) Basic
(d) Corrosive
Answer:
(c) Basic
Explanation: The toothpaste commonly used is alkaline or basic in nature as they contain mild bases such as sodium fluoride or sodium bicarbonate in their composition.
The base reacts with the acid formed during bacterial action in the mouth and neutralises its bad effects. Thus, preventing tooth decay. So, they can neutralize the effect of extra acids being formed in the mouth cavity which are mainly responsible for tooth decay.
Related Theory
When we eat sweet things, the pH of our mouth falls below 5.5 (moderately acidic) as the oral bacteria release acid while acting on sugars present in our food. The acidic conditions are capable of corroding the enamel which is made up of calcium phosphate. This causes the tooth to decay.
Question 11.
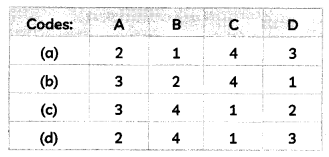
Match the chemical substances given in Column I with their appropriate application given in Column II:
| Column I | Column II |
| (A) Bleaching Powder | (1) Preparation of glass |
| (B) Baking Soda | (2) Production of H2 and Cl2 |
| (C) Washing Soda | (3) Decolourisation |
| (D) Sodium Chloride | (4) Antacid |
Answer:
Question 12.
Which of the following phenomena occur, when a small amount of acid is added to water?
(I) Ionisation
(II) Neutralisation
(III) Dilution
(IV)Salt formation
(a) (I) and (II)
(c) (II) and (III)
(b) (I) and (III)
(d) (II) and (IV)
Answer:
Question 10.
To protect against tooth decay we are advised to brush our teeth regularly. The nature of the toothpaste commonly used is:
(a) Acidic
(b) Neutral
(c) Basic
(d) Corrosive
Answer:
(c) Basic
Explanation: The toothpaste commonly used is alkaline or basic in nature as they contain
Question 13.
A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue:
(a) Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Answer:
(d) An antacid
Explanation: pH paper gives greenish-blue colour in a weak alkaline medium so antacid [Mg(OH)2] which is an alkaline compound will show greenish-blue color on pH paper.
Related Theory
Lemon fruit contains citric acid, vinegar has acetic acid and common salt is the neutral salt the clear super natant solution turns the pH paper yellowish-orange means the given sample of soil is acidic so it can be neutralised by base/alkaline solution.
Question 14.
Which of the following is not a mineral acid?
(a) Hydrochloric acid
(b) Citric acid
(c) Sulphuric acid
(d) Nitric acid
Answer:
Question 15.
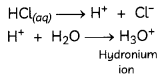
Which of the following is/are true when HCl(g) is passed through water?
(I) It does not ionise in the solution as it is a covalent compound.
(II) It ionises in the solution.
(III) It gives both hydrogen and hydroxyl ions in the solution.
(IV) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule.
(a) Only (I)
(b) Only (III)
(c) (II) and (IV)
(d) (III) and (IV)
Answer:
(c) (II) and (IV)
Explanation: HCl, is a polar covalent compound, easily ionises in water to form hydronium (H3O+) and chloride ions (Cl–). HCl (a strong acid) ionises completely in water to produce H+ and Cl– ions. H+ ion combines with water molecules to produce hydronium ions.
Question 16.
Which among the following is not a base?
(a) NaOH
(b) KOH
(c) NH4OH
(d) C2H5OH
Answer:
(d) C2HsOH
Explanation: C2H5OH is not a base. C2H5OH is an organic compound with -OH functional group that is known as alcohol. It cannot give OH– ions in its solution. It cannot dissociate ions in a solution. Thus, it cannot be a basic compound.
Question 17.
Identify the correct representation of reaction occurring during the chloralkali process.
(a) 2NaCl(l) + 2H2O(l) → 2NaOH(l) + Cl2(g) + H2(g)
(b) 2NaCl(l) + 2H2O(aq) → 2NaOH(aq) + Cl2(g) + H2(aq)
(c) 2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(aq) + H2(aq)
(d) 2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
Answer:
Question 18.
Which of the following statements is true for acids?
(a) Bitter and change red litmus to blue.
(b) Sour and change red litmus to blue.
(c) Sour and change blue litmus to red.
(d) Bitter and change blue litmus to red.
Question 19.
Zinc granules on treating with an acid X, form zinc sulphate (ZnSO4) salt along with the evolution of a gas Y, which burns with a pop sound when brought near to a burning candle. Identify acid X and gas evolved Y.
(a) X-sulphuric acid and Y-oxygen gas
(b) X-hydrochloric acid and Y-oxygen gas
(c) X-sulphuric acid and Y-hydrogen gas
(d) X-hydrochloric acid and Y-hydrogen gas [Diksha]
Answer:
(c) X-sulphuric acid and Y-hydrogen gas
Explanation: When an acid reacts with a metal, hydrogen gas is liberated. In the given reaction, when dilute sulphuric acid reacts with zinc granules, hydrogen gas is liberated and zinc sulphate solution is formed:
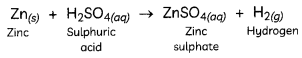
The presence of hydrogen gas is tested by bringing a lighted candle near it. When a lighted candle is brought near the test tube containing hydrogen gas, it burns with a ‘pop’ sound making a little explosion.
Hence, acid X is sulphuric acid and gas Y is hydrogen gas.
Question 20.
Which of the following solutions in water does not conduct electricity?
(a) Hydrochloric acid
(b) Sodium chloride
(c) Glucose
(d) Sulphuric acid [Diksha]
Answer:
(c) Glucose
Explanation: The aqueous solution of an acid conducts electricity because of the presence of charged particles called ‘ions’ in it. When hydrochloric acid (HCl) is dissolved in water, its aqueous solution contains hydrogen ions (H+) and chloride ions (Cl–). These ions carry electric currents. So, due to the presence of H+ and Cl– ions, a solution of hydrochloric acid conducts electricity.
On the other hand, the hydrogen-containing compound such as glucose is not categorized as acid because it does not produce hydrogen ions or some other ions when dissolved in water and hence does not conduct electricity. Therefore, the answer is glucose.
Question 21.
Which of the following will turn phenolphthalein pink?
(a) NaOH(aq)
(b) HCl(aq)
(c) CH3COOH(aq)
(d) H2O [Diksha]
Answer:
Question 22.
Which one of the following is not a use of washing soda:
(a) Sodium carbonate (washing soda) is used in glass, soap and paper industries.
(b) It is used in the manufacture of sodium compounds such as borax.
(c) Sodium carbonate can be used as a clean¬ing agent for domestic purposes.
(d) It is used for disinfecting water.
Answer:
(d) It is used for disinfecting water.
Explanation: The compound that is used for disinfecting water is bleaching powder. How¬ever, washing soda is used for removing the permanent hardness of water.
Question 23.
The approximate pH values of four salts is given below. Select the row(s) containing the correct information.
| Name of Salt | PH |
| (I) Potassium Sulphate | 10 |
| (II) Ammonium nitrate | 5 |
| (III) Sodium acetate | 3 |
| (IV) Sodium hydrogen carbonate | 8 |
(a) Both (I) and (II)
(b) Both (II) and (III)
(c) Both (III) and (IV)
(d) Both (II) and (IV)
Answer:
Question 24.
Zinc granules on treating with a substance X, form a salt sodium zincate along with the evolution of a gas Y which burns with a pop sound when brought near a burning candle. Identify the substance X and gas evolved Y.
| X | Y |
| (a) Acetic acid | Hydrogen |
| (b) Sodium hydroxide | Hydrogen |
| (c) Sodium hydroxide | Oxygen |
| (d) Zinc hydroxide | Hydrogen |
Answer:
(b) X is Sodium hydroxide and Y is hydrogen.
Explanation: When zinc granules react with sodium hydroxide, salt sodium zincate is formed along with hydrogen gas, which burns with a pop sound.
The equation of the reaction taking place is:
2NaOH(aq) + Zn(s) → Na2ZnO2(aq) + H2(g)
Question 25.
Which of the following salts belong to the same family of salts?
(I) sodium chloride and sodium acetate
(II) calcium sulphate, magnesium sulphate
(III) sodium carbonate and sodium hydrogen -carbonate
(IV) sodium chloride and magnesium sulphate
(a) Both (I) and (II)
(b) Both (I) and (III)
(c) (I), (II) and (III)
(d) (I), (III) and (IV)
Answer:
Question 26.
A student noted his observations regarding the acidic or basic nature of salts as below:
(I) Sodium chloride is a neutral salt
(II) Ammonium chloride is a basic salt
(III) Sodium carbonate is a neutral salt
(IV) Copper sulphate is an acidic salt Select the incorrect observations:
(a) Both (I) and (III)
(b) Both (II) and (III)
(c) Both (I) and (IV)
(d) Both (II) and (IV)
Answer:
(b) Both (II) and (III)
Explanation: The salts of strong acids and strong bases give neutral solutions. Therefore, sodium chloride is a neutral salt as it is obtained from sodium hydroxide (a strong base) and hydrochloric acid (a strong acid).
The salts of strong acids and weak bases give acidic solutions. Therefore, ammonium chloride and copper sulphate are acidic salts as ammonium chloride is obtained from ammonium hydroxide (a weak base) and hydrochloric acid (a strong acid), and copper sulphate is obtained from copper hydroxide (a weak base) and sulphuric acid (a strong acid).
The salts of weak acids and strong bases give basic solutions. Therefore, sodium carbonate is a basic salt as it is obtained from carbonic acid (a weak acid) and sodium hydroxide(a strong base).
Assertion Reasoning questions Class 10 Science Chapter 2
For the following questions, two statements are given – one labelled Assertion (A) and the other labeled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both (A) and (R) are true and (R) is the correct explanation of the (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Question 27.
Assertion,(A): HCl gas does not change the colour of dry blue litmus paper.
Reason (R): Acids always produce hydrogen ions.
Answer:
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the (A).
Explanation: HCl gas does not change the colour of dry litmus paper but changes colour of moist litmus paper as acids produce hydrogen ions only in solution.
Question 28.
Assertion (A): Generally, the colour of indicators changes in particular pH range.
Reason (R): Indicators are weak acids or weak base and exhibit different colours in molecular form and ionic form.
Answer:
(a) (A) and (R) are true and (R) is the correct explanation of the (A).
Explanation: Since indicators are weak acids or weak bases, their percentage of existence in molecular state and in ionized state depends on the strength of acidic/basic/neutral solution to which they are added. Hence their colours change at a particular pH range.
Question 29.
Assertion (A): Zinc reacts with sodium hydroxide solution and hydrogen gas is evolved.
Reason (R): All metals react with bases to evolve hydrogen gas.
Question 30.
Assertion (A): While diluting an acid, water is slowly added to acid with constant stirring.
Reason (R): The process of dissolving an acid in water is a highly exothermic reaction.
Question 31.
Assertion (A): Metal oxides are acidic in nature.
Reason (R): Calcium hydroxide reacts with carbon dioxide to form a salt and water.
Answer:
(d) (A) is false, but (R) is true.
Explanation: The reaction of calcium hydroxide with carbon dioxide to form a salt and water is similar to the reaction between an acid and a base. Therefore, non-metal oxides are acidic in nature.
Question 32.
Assertion (A): When copper sulphate crystals are heated in a dry boiling tube, they turn white.
Reason (R): Water of crystallization is the number of water molecules present in one formula unit of a salt.
Answer:
(b) Both (A) and (R) are true but (R) is not the correct explanation of (A).
Explanation: Copper sulphate crystals that seem to be dry contain water of crystallisation. When we heat the crystals, this water is removed and the salt turns white.
CUSO4.5H2O → CuSO4 + 5H2O
Question 33.
Assertion (A): When copper oxide is added to dilute hydrochloric acid, the colour of the solution becomes blue-green.
Reason (R): Copper (II) chloride is formed.
Answer:
(a) (A) and (R) are true and (R) is the correct explanation of the (A).
Explanation: When copper oxide is added to dilute hydrochloric acid, the colour of the solution becomes blue-green due to the formation of Copper (II) chloride, which is blue-green in colour.