S Chand Biology Class 10 Solutions Model Test Paper 2
Question 1.
How is the increase in demand for energy affecting our environment adversely?
Answer:
As the population increases, so does their demand for energy. More and more of fossil fuels are being used to generate electricity and for transportation purposes. Use of fossil fuels in large amounts, add heat and pollution to the environment thus affecting the climatic conditions and the environment adversely.
Question 2.
Balance the following chemical equation.
\(\mathrm{FeSO}_{4} \stackrel{\text { Heat }}{\longrightarrow} \mathrm{Fe}_{2} \mathrm{O}_{3}+\mathrm{SO}_{2}+\mathrm{SO}_{3}\)
Answer: Balanced chemical equation.
\(2 \mathrm{FeSO}_{4} \stackrel{\mathrm{Heat}}{\longrightarrow} \mathrm{Fe}_{2} \mathrm{O}_{3}+\mathrm{SO}_{2}+\mathrm{SO}_{3}\)
Question 3.
Draw ray diagrams to represent the nature, position and relative size of the image formed by a convex lens for the object placed.
(a) at 2F1
(b) between F1 and the optical center O of the lens
Answer:
(a) When the object is at 2F1
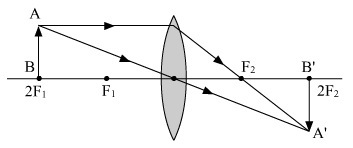
Nature of the image formed. Real and inverted
Position of the image formed. At 2F2
Size of the image formed. Same as that of the object
(b) When the object is placed between the focus F1 and optical center O.
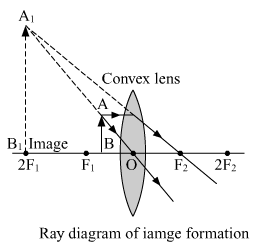
Nature of the image formed. Virtual and erect
Position of the image formed. Same side of the object, beyond the 2F1
Size of the image formed. Enlarged
Question 4.
Give an example of a decomposition reaction. Describe an activity to illustrate such a reaction by heating.
Answer:
A decomposition reaction is a chemical reaction in which a single compound breaks down to simpler products.
Example. Decomposition of ferrous sulphate crystals.
![]()
Heat the ferrous sulphate crystals, the colour of ferrous sulphate crystals is green. On heating, the ferrous sulphate crystals lose their water molecules. Therefore, the colour of the crystals change.
Question 5.
Write one function each of the following components of the transport system in human beings.
(a) Blood vessels
(b) Blood platelets
(c) Lymph
(d) Heart
Answer:
(a) Blood vessels help in transporting oxygenated and deoxygenated blood to different parts of the body.
(b) Platelets help in the process of blood clotting.
(c) Lymph carries digested and absorbed fats from the intestine. It also provides immunity to the body.
(d) Heart acts as a pump and helps circulate blood throughout the body.
Question 6.
Name two metals which react violently with cold water. Write any three observations you would make when such a metal is dropped into water. How would you identify the gas evolved, if any, during the reaction?
Answer:
Sodium and potassium are two metals that react violently with cold water.
Observations made when such a metal is dropped in water.
- The reaction is highly exothermic, i.e. a huge amount of heat is produced.
- Hydrogen gas is evolved in the reaction
- Little explosions take place because the heat produced in the chemical reaction burns the hydrogen gas.
Hydrogen gas can be identified by the pop sound produced during the reaction of metal with cold water.
Question 7.
(a) Why are covalent compounds generally poor conductors of electricity?
(b) Name the following compound.
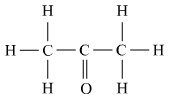
(c) Name the gas evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas?
Answer:
(a) As covalent bonds do not break easily (dissociate), so they do not form ions which help in conduction of electricity. Therefore, covalent compounds don’t conduct electricity as they don’t have free electrons or ions.
(b) The given compound contains 3 carbons and a ketone functional group so it would be called as ‘propanone’.
(c) When ethanoic acid reacts with sodium carbonate, sodium ethanoate, carbon dioxide, and water are formed.
\(2 \mathrm{CH}_{3} \mathrm{COOH}+\mathrm{Na}_{2} \mathrm{CO}_{3} \rightarrow \mathrm{CH}_{3} \mathrm{COONa}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}\)
To test the presence of formed gas, we pass this gas from lime water. Upon reaction, lime water turns milky, which confirms the presence of carbon dioxide in the reaction.
Question 8.
(a) What is the difference between washing soda and baking soda?
(b) Write the chemical formula for bleaching powder. How is bleaching powder prepared? For what purpose is it used in paper factories?
Answer:
(a) Washing soda: Chemical formula of washing soda is Na2CO3. 10H2O Washing soda used as a cleansing agent in domestic household purposes.
Baking Soda: Chemical formula of baking soda is NaHCO3. Baking soda is used as an antacid to remove acidity in the stomach.
(b) The chemical formula of bleaching powder is CaOCl2.
Bleaching powder is prepared by passing chlorine gas over dry calcium hydroxide.
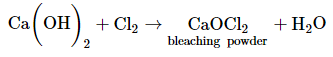
Bleaching powder is used in paper factories for bleaching wood pulp, as bleaching powder is a strong oxidizing agent.
Question 9.
In the electrolysis of acidified water.
(a) Name the gas collected at (i) anode, and (ii) cathode
(b) Why is the volume of gas collected at one electrode double than that at the other electrode?
(c) What would happen if dilute sulphuric acid is not added to water?
Answer:
(a) Electrolysis of acidified water.
At cathode: 2H+ + 2e– → H2
At anode: OH– → OH + e–
4OH → 2H2O2
Hence, the gas produced at cathode is hydrogen and the gas produced at anode is oxygen.
(b) A water molecule contains two atoms of hydrogen and one atom of oxygen. Therefore, during the electrolysis of water, the ratio of the amount of hydrogen gas and oxygen gas produced is 2.1. Hence, we can say that the volume of gas collected at one electrode is double than that at the other electrode
(c) As electrolysis of water is a slow process, to increase the rate of electrolysis we add dilute sulphuric acid.
Dilute sulphuric acid increases the rate of breakdown of ions.
Question 10.
Two lamps, one rated 40 W at 220 V and the other 60 W at 220 V, are connected in parallel to the electric supply at 220 V.
(a) Draw a circuit diagram to show the connections.
(b) Calculate the current drawn from the electric supply.
(c) Calculate the total energy consumed by the two lamps together when they operate for one hour.
Answer:
(a)
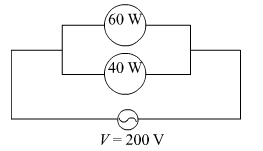
(b) Using, I = \(\frac{P}{V}\)
Current drawn by 60 W lamp is, I = \(\frac{220}{60}\) = 3.67 A
Current drawn by 40 W lamp is, I = \(\frac{220}{40}\) = 5.5 A
So, the net current drawn by the combination is = 3.67 A + 5.5 A = 9.17 A
(c) Net power of the combination = 60 W + 40 W = 100 W
So, the energy consumed in 1 h = E = P × t = 100 × 1 × 60 × 60 J = 360000 J
Question 11.
(a) Why is a series arrangement not used for connecting domestic electrical appliances in a circuit?
(b) Draw a diagram to show the magnetic field lines around a bar magnet. List any two properties of magnetic field lines.
Answer:
(a) A series arrangement not used for connecting domestic electrical appliances in a circuit because if any one of the components connected in series stops working or get damaged, then the whole circuit would break up and no current will flow further in the circuit and none of the other devices would work.
(b)
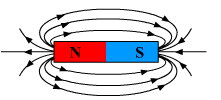
Two properties of magnetic field lines.
- They originate from the north pole of the magnet and end at the south pole of the magnet.
- They do not intersect each other.
Question 12.
Name the electric device which converts mechanical energy into electrical energy. Draw the labelled diagram and explain the principle involved in this device.
Answer:
An electric generator converts mechanical energy into electrical energy.
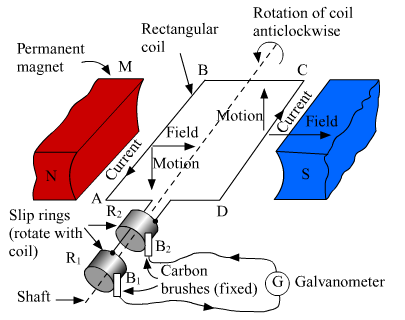
MNST → Rectangular coil
A and B → Brushes
C and D → Two slip rings
X → Axle, G → Galvanometer
The principle of working of an electric generator is that when a loop is moved in a magnetic field, an electric current is induced in the coil. It generates electricity by rotating a coil in a magnetic field. The above figure shows a simple AC generator.
Question 13.
Write any three differences between aerobic and anaerobic respiration.
Answer:
|
Aerobic Respiration
|
Anaerobic respiration |
| It involves the complete breakdown of glucose into CO2 and H2O. | It involves the partial breakdown of glucose. |
| It occurs in the presence of oxygen. | It occurs in the absence of oxygen. |
| A net gain of 36 molecules of ATP occurs. | A net gain of only 2 molecules of ATP occurs. |
| Occurs in plants and animals (eukaryotes). | Occurs in human muscle cells. bacteria, yeast etc. |
Question 14.
How is ozone formed in the upper atmosphere? Why is damage to ozone layer a cause of concern to us? What causes this damage?
Answer:
Ultraviolet radiations split the oxygen molecules present in stratosphere into free oxygen atoms. These free oxygen atoms then combine with molecular oxygen to form ozone.
O2 → O + O
O2 + O → O3
Ozone layer is a protective layer which protects the earth from harmful UV radiations. Any damage to the ozone layer would result in UV rays entering the earth’s atmosphere. These UV rays can cause diseases such as skin cancer and cataract.
They can also adversely affect the crops.
The ozone layer is damaged as a result of chlorofluorocarbons. These are often used in refrigerators, air conditioners and fire extinguishers.
Question 15.
(a) State one difference between arteries and veins.
(b) Name any two sexually transmitted diseases. What advice is given to prevent them?
Answer:
(a)
| Arteries | Veins |
| They carry oxygenated blood from the heart to the other parts of the body. The pulmonary artery is an exception as it carries deoxygenated blood from the heart to lungs. |
They carry deoxygenated blood from the body to the heart. The pulmonary vein is an exception as it carries oxygenated blood from the heart to lungs. |
(b) Syphilis and AIDS are two sexually transmitted diseases. Such diseases can be prevented by the use of condoms.
Condoms are made of thin rubber and are used to cover the penis and the vagina in males and females, respectively.
Question 16.
(a) What is meant by the dispersion of white light? Describe the formation of the rainbow in the sky with the help of a diagram.
(b)What is hypermetropia? Draw ray diagrams to show the image formation of an object by.
(i) Hypermetropic eye
(ii) Correction made with a suitable lens for the hypermetropic eye.
Answer:
(a) The splitting of white light into seven colors on passing through a transparent glass prism is called dispersion of white light.
A rainbow is formed in the sky because of the dispersion of sunlight by millions of water droplets in the atmosphere. Every single water droplet behaves like a tiny glass prism that splits the white light into different colors. Different colors bend at different angles on passing through the water droplets, thus separating the white light into a natural spectrum of seven colors, as Violet, Indigo, Blue, Green, Yellow, Orange, and Red. The diagram below shows the formation of a rainbow.
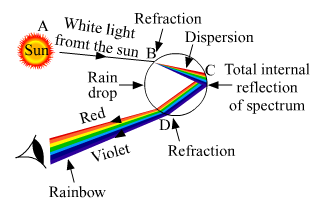
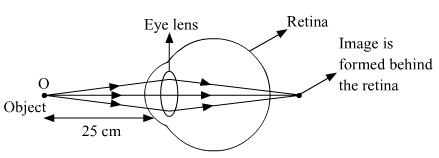
(b) Hypermetropia or long-sightedness is a common eye defect, in which a person is not able to nearby objects whereas the farther objects appear clear to the person.
(i) Hypermetropic eye.
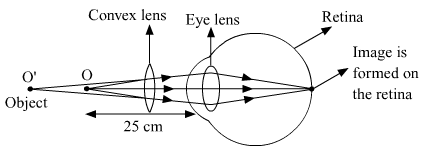
(ii) Correction made with a convex lens for the hypermetropic eye.
Question 17.
(a) Give reasons for the following.
(i) Colour of the clear sky is blue.
(ii) The sun can be seen about two minutes before the actual sunrise.
(iii) We cannot see an object clearly if it is placed very close to the eyes.
(b) What is Presbyopia? Write two causes of this defect.
Answer:
(a) (i) The sky appears blue when viewed from Earth, because of the scattering of the light. When sunlight strikes molecules in our atmosphere, the light is redirected in many directions. The blue light is scattered more than the red light because blue color has the smallest wavelength i.e. comparable to the size of the particles present in our atmosphere. So, it is scattered the most, causing the sky to be blue.
(ii) The Sun is visible to us two minutes before the sunrise and two minutes after the sunset because of the bending of the light due to atmospheric refraction. The light from the Sun gets refracted by the atmospheric layers and reaches the observer. This causes the observer to think that the light is coming straight from the Sun.
(iii) If an object is placed very close to the eyes, the light reflected from it does not fall on the retina to form a clear image. The minimum distance for seeing an object clearly is 25 cm for the human eye.
(b) Presbyopia is a common defect of vision, which generally occurs at old age. A person suffering from this type of defect of vision cannot see the nearby objects clearly and distinctively. A presbyopic eye has its near point greater than 25 cm and it gradually increases as the eye becomes older.
Presbyopia is caused by the.
1. The weakening of the ciliary muscles.
2. Reduction in the flexibility of the eye lens.
Question 18.
(a) Why do we classify elements?
(b) What were the two criteria used by Mendeleev in creating his Periodic Table?
(c) Why did Mendeleev leave some gaps in his Periodic Table?
(d) In Mendeleev’s Periodic Table, why was there no mention of Noble gases such as Helium, Neon and Argon?
(e) Would you place the two isotopes of chlorine, Cl-35 and Cl-37 in different slots because of their different atomic masses or in the same slot because their chemical properties are the same? Justify your answer.
Answer:
(a) The need to classify elements into families with similar properties arose because, lacking this basic tool, the chemist had to deal with each of the 45 or so known elements one at a time, as if there were no rhyme or reason to them.
(b) Two criteria used by Mendeleev in creating his periodic table.
(i)Mendeleev’s periodic table was based on the observation that the properties of elements are the function of their atomic masses. This means that if elements are arranged in the increasing order of their atomic masses, then their properties get repeated after regular intervals.
(ii) Relative atomic mass and similarity of chemical properties.
(c) As at the time of Mendeleev some elements are not discovered, so he realized this fact and left gaps in his table for such elements and also predicted their properties.
(d) Because at the time of Mendleeve these elements are not discovered, so he did not mention about them.
(e) In the modern periodic table, all isotopes are placed in the same position as in modern periodic table is based on the atomic number not on atomic masses.
Question 19.
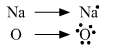
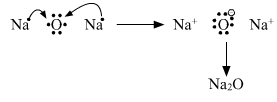
(a) Show the formation of Na2O by the transfer of electrons between the combining atoms.
(b) Why are ionic compounds usually hard?
(c) How is it that ionic compounds in the sold state do not conduct electricity but they do so when in molten state?
(d) What type of chemical bonds are present in CCl4?
(e) What type of chemical bonds are present in MgCl2?
Answer:
(a) Electronic configuration of Na2O.
Formation of ionic bond.
(b) Ionic compounds are usually hard because of inter-atomic attractive forces between them. Positively charged ion and negatively charged ion are strongly attracted towards each other and their lattice enthalpy increases.
(c) Electricity can be conducted only in the presence of free electrons/ions. In the solid state, there will be no free electrons but in the molten state, ions are free to conduct electricity.
(d) In carbon tetrachloride, four single bonds are formed. Hence, covalent bonding is present in CCl4.
(e) In magnesium chloride, magnesium will lose its electrons and chlorine will accept those electrons to form magnesium chloride. Hence. ionic bonding is present in magnesium chloride.
Question 20.
(a) Draw the structure of a neuron and label the following on it.
Nucleus, Dendrite, Cell body and Axon
(b) Name the part of neuron.
(i) where information is acquired.
(ii) through which information travels as an electrical impulse.
Answer:
(a)
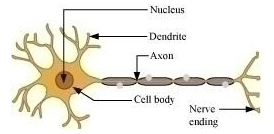
(b)
(i) Dendrites acquire stimulus or information from the other cells.
(ii) Axon carries away information from the cell body to the dendrite of the next neuron.
Question 21.
(a) What is (i) phototropism, and (ii) geotropism? With labelled diagrams, describe an activity to show that light and gravity change the direction that plant parts grow in.
(b) Mention the role of each of the following plant hormones.
(i) Auxin
(ii) Abscisic acid
Answer:
(a) (i) The bending of shoots or other plant parts in response to light is known as phototropism.
(ii) The bending or growth of roots towards gravity is known as geotropism.
Activity –
Fill a conical flask with water and then cover the neck of the flask with a wire mesh. Place few germinating seeds on the wire mesh. Then, take a cardboard box that is open from one side. Place the flask in the box and keep this setup near a window. After 3-4 days, you will notice the shoots start bending towards light, while the roots bend away from light.
(b) (i) Auxin helps cells grow longer.
(ii) Abscisic acid inhibits growth and causes wilting of leaves.
Question 22.
(a) How will you test for the gas which is liberated when hydrochloric acid reacts with an active metal?
(b) Name any two active metals which can be used with hydrochloric acid to liberate the same gas.
Answer:
(a) When hydrochloric acid is treated with an active metal then hydrogen gas is released.
\(2 \mathrm{M}+2 \mathrm{HCl} \rightarrow 2 \mathrm{MCl}+\mathrm{H}_{2}\)
When this hydrogen gas comes in contact with burning match stick then the gas burns with a pop sound.
(b) Sodium and potassium are two active metals which can be used with hydrochloric acid to liberate the hydrogen gas.
Question 23.
Sunita performs two sets of experiments to study the formation of lather(foam) by using liquid soap.
Set 1 . She takes 10 ml of distilled water in test tube ‘A’, adds 5-6 drops of liquid soap in it and shakes the test tube vigorously.
Set 2 . She takes 10 ml of distilled water in test tube ‘B’, adds 5-6 drops of liquid soap in it alongwith half spoonful of CaSO4, and shakes the test tube vigorously.
(a) In which test tube, A or B, less lather is formed? Why?
(b) In which test tube, A or B, more lather is formed? Why?
Answer:
(a) Test tube “B” has less lather due to the presence of calcium ions. Due to the presence of calcium ions water become hard and in hard water, lather formation decreases.
(b) Test tube “A” has more lather because it is soft water. In soft water, lather formation increases and cleansing action increases.
Question 24.
(a) Copy this figure in your answer-book and show the direction of the light ray after reflection.
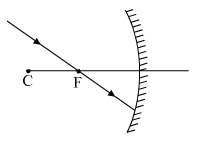
(b) What type of mirror is shown in the above figure. a converging mirror or a diverging mirror?
Answer:
(a) The ray will get reflected from the mirror and will go parallel to the principal axis of the mirror.
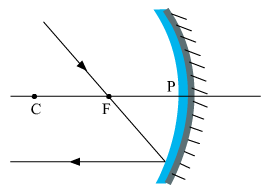
(b) The mirror shown in the given figure is a converging mirror or a concave mirror.
Question 25.
A student is performing an experiment to verify Ohm’s Law in the laboratory. The ammeter given to him for this purpose has 10 divisions between 0 and 0.5 A on its scale. If the pointer shows 22 divisions on the ammeter scale while taking a reading, what is the value of current according to this position of pointer?
Answer:
The given ammeter has 10 division between 0 and 0.5 A.
Value of one division on the ammeter scale = 0.05 A
If the pointer shows 22 division for the main scale reading, then the actual reading = 22 × 0.05 A = 1.1 A
Hence, the value of the current according to the given pointer is 1.1 A.
Question 26.
In the test tubes A and B shown below, yeast was kept in sugar solution. Which products of respiration would you expect in tubes A and B?
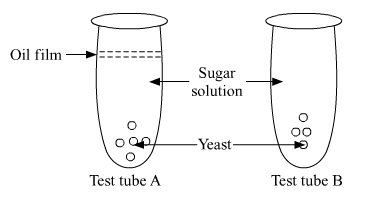
Give reason for your answer.
Answer:
In test tube A, anaerobic respiration will occur. The top of test tube A is covered with a film of oil which means no oxygen would be available to the yeast cells for respiration. In this case, the product of respiration will be ethanol, carbon dioxide and energy.
Glucose → Pyruvate → 2C2H5OH + 2CO2 + 2ATP
In test tube B, aerobic respiration will occur. The products of aerobic respiration will be carbon dioxide, water and energy.
Glucose → Pyruvate → 6CO2 + 6H2O + 38ATP
Question 27.
Before testing the leaf for starch at the end of the experiment “Light is necessary for photosynthesis”.
(a) In which liquid the leaf should be boiled?
(b) Why should the leaf should be boiled in this liquid?
Answer:
(a) The leaf should be boiled in alcohol (ethanol).
(b) The leaf is boiled in alcohol to destarch it (remove the chlorophyll from the leaf) as the chlorophyll interferes with the testing of starch with iodine.