Combustion:
Carbon, in all its allotropic forms, burns in oxygen to give carbon dioxide along with the release of heat and light. Most carbon compounds also release a large amount of heat and light on burning.
- C + O2 → CO2 + heat and light
- CH4 + 2O2 → CO2 + 2H2O + heat and light
- CH3CH2OH + O2 → CO2 + H2O + heat and light
Example 1.
Case-Based:
Two students performed the following activities to study the combustion of hydrocarbons.
The first student took, some carbon compounds (naphthalene, camphor, alcohol) one by one on a spatula and burned them with the teacher’s assistance. He observed the nature of the flame and whether smoke is produced. He then placed a metal plate above the flame and noted down his observations.
The second student lighted a bunsen burner and adjusted the air hole at the base to get different types of flames/presence of smoke.
(A) The observations recorded by the first student are given below:
(I) Naphthalene burns with a yellow flame
(II) Camphor burns with a blue flame
(III) Alcohol burns with a blue flame
(IV) No smoke is produced when these substances are burnt Which of the observations are correct?
(a) Both (I) and (II)
(b) Both (II) and (III)
(c) Both (I) and (III)
(d) (I), (II) and (IV)
Answer:
(c) Both (I) and (III)
Explanation: When alcohol, camphor and naphthalene are taken in a spatula and burnet separately, the yellow coloured flame is observed in the case of camphor and naphthalene as they both are unsaturated hydrocarbons. Whereas, alcohol burns with a blue flame as it is a saturated hydrocarbon
(B) The second student recorded his observations which are given as follows:
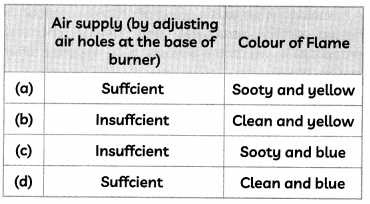
Select the correct observation.
Answer:
(d) Air supply: Sufficient: Colour of flame: Clean and blue
Explanation: When the air supply is sufficient then, the burns with a clean blue flame as it undergoes complete combustions. However, when the air supply is insufficient, the fuel burns with a sooty yellow flame as all the carbon particles do not burn completely. So, the unburnt carbon particles deposit as soot.
(C) Which out of the following hydrocarbons will give a clean flame when burnt: C4H10 or C4H8?
Answer:
The hydrocarbon C4H10 or butane will give a clean flame when burnt as it is an aLkane, which is a saturated hydrocarbon containing only single covalent bonds between carbon atoms.
(D) Do saturated hydrocarbons always burn with a blue flame?
Answer:
No, saturated hydrocarbons burn with a blue flame in sufficient supply of oxygen and burn with a yellow sooty flame when supply of oxygen is insufficient.
(E) Assertion (A): Burning of camphor results in a sooty deposit on a metal plate kept over the flame.
Reason (R): Saturated hydrocarbons always burn with a sooty flame.
(a) Both (A) and (R) are true and (R) is the correct explanation of the (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Answer:
(c) (A) is true, but (R) is false.
Explanation: When camphor is burned and a metal plate is kept over the flame, it burns with a yellow sooty flame and a sooty deposit is formed on the metal plate. This is because camphor is an unsaturated hydrocarbon and all unsaturated hydrocarbons burn with a sooty flame due to the incomplete combustion of carbon particles.
Saturated hydrocarbons burn with a clean flame insufficient supply of oxygen.
Colour of Flame on Burning Hydrocarbons
Saturated hydrocarbons will generally give a clean blue flame while unsaturated carbon compounds will give a yellow flame with lots of black smoke.
However, Limiting the supply of air results in incomplete combustion of even saturated hydrocarbons giving a sooty flame.
The gas/kerosene stove used at home has inlets for air so that a sufficiently oxygen-rich mixture is burnt to give a clean blue flame.
If the bottoms of cooking vessels are getting blackened, it means that the air holes are blocked and fuel is getting wasted.
Fuels such as coal and petroleum have some amount of nitrogen and sulphur in them. Their combustion results in the formation of oxides of sulphur and nitrogen which are major pollutants in the environment.
Burning substances and flames: A flame is only produced when gaseous substances burn. When wood or charcoal is ignited, the volatile substances present vapourise and burn with a flame in the beginning.
A luminous flame is seen when the atoms of the gaseous substance are heated and start to glow. The colour produced by each element is a characteristic property of that element.
Formation of Coal and Petroleum
Coal and petroleum have been formed from biomass which has been subjected to various biological and geological processes. Coal is the remains oftrees, ferns, and other plants that lived milLions of years ago. These were crushed into the earth, perhaps by earthquakes or volcanic eruptions. They were pressed down by layers of earth and rock. They slowly decayed into coal.
Oil and gas are the remains of millions of tiny plants and animals that lived in the sea. When they died, their bodies sank to the sea bed and were covered by silt. Bacteria attacked the dead remains, turning them into oil and gas under the high pressures they were being subjected to.
Oxidation
Oxidation is the reaction in which oxygen is added and hydrogen is removed from alcohol. When alcohol is heated with an oxidizing agent such as alkaline potassium permanganate or acidified potassium dichromate, they undergo oxidation and form a carboxylic acid.
![]()
AlkaLine potassium permanganate or acidified potassium dichromate are known as oxidising agents as they oxidise alcohols to acids, that is, add oxygen to the starting material
Addition Reaction
The reaction in which an atom or a group of atoms is added to a molecule is known as an addition reaction. Unsaturated hydrocarbons add hydrogen in the presence of catalysts such as palladium or nickel to give saturated hydrocarbons. This reaction is commonly used in the hydrogenation of vegetable oils using a nickel catalyst. Vegetable oils generally have long unsaturated carbon Chains while animal fats have saturated carbon chains.
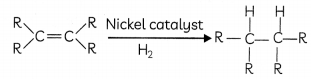
Hydrogenation of Vegetable Oils
Oils (such as vegetable, olive, sunflower) are liquids at room temperature. In the food industry, hydrogen is added to oils (in a process called hydrogenation) to make them more solid, or ‘spreadable’. The hydrogenation of oils helps to prolong the sheLf-life of the food and maintain flavour stability.
Since the process of hydrogenation adds hydrogen atoms to oil, it will reduce the number of unsaturated fatty acids and increase the number of saturated fatty acids in the oil.
Animal fats generally contain saturated fatty acids which are said to be harmfuLfor health. Oils containing unsaturated fatty acids should be chosen for cooking.
Example 2.
Which of the following hydrocarbons undergo additional reactions:
C2H6, C3H8I C3H6I C2H2 and CH4.
Answer:
Unsaturated hydrocarbons (alkenes and alkynes) undergo additional reactions as they have double and triple covalent bonds which are the site of chemical reactivity. The general formula of alkenes is CnH2n and of alkynes is CnH2n-2.
Out of the given hydrocarbons, C2H6, C3H8 and CH4 are alkanes as they have the general formula CnH2n+2. Hence they will not undergo addition reaction.
C3H6 is an alkene (ethene) and will undergo additional reaction.
C2H2 is an alkyne (ethyne) and will also undergo additional reaction.
Example 3.
Give a test that can be used to differentiate chemically between butter and cooking oil.
Answer:
Butter contains saturated compounds and cooking oil contains unsaturated compounds. As unsaturated hydrocarbons undergo addition reaction whereas saturated hydrocarbons do not undergo addition reaction, we will use bromine water test to differentiate chemically between butter and cooking oil.
Bromine water (reddish-brown in colour) is decolourized by unsaturated hydrocarbons as they will undergo addition reaction with bromine.
CH2 = CH2 + Br2 → CH2Br—CH2Br
Whereas, the brown colour of bromine will be retained on reacting with a saturated hydrocarbon. So, when a small amount of butter and cooking oil are taken and treated with bromine water, cooking oil wilL decolourize the bromine water whereas butter will not have any effect on it.
Substitution Reaction
The reaction in which an atom replaces another atom or a group of atoms from a molecule is called a substitution reaction.
Saturated hydrocarbons are fairly unreactive and are inert in the presence of most reagents. In the presence of sunlight, chlorine is added to hydrocarbons in a very fast reaction. Chlorine can replace the hydrogen atoms one by one. It is called a substitution reaction because one type of atom or a group of atoms takes the place of another. A number of products are usually formed with the higher homologues of alkanes.
CH4 + Cl2 → CH3Cl + HCl (In the presence of sunlight)