Practicing the CBSE Sample Papers for Class 12 Chemistry with Solutions Set 4 allows you to get rid of exam fear and be confident to appear for the exam.
CBSE Sample Papers for Class 12 Chemistry Set 4 with Solutions
Time : 3 Hours
Maximum Marks: 70
General Instructions :
Read the following instructions carefully.
- There are 35 questions in this question paper with internal choice.
- SECTION A consists of 18 multiple-choice questions carrying 1 mark each.
- SECTION B consists of 7 very short answer questions carrying 2 marks each.
- SECTION C consists of 5 short answer questions carrying 3 marks each.
- SECTION D consists of 2 case-based questions carrying 4 marks each.
- SECTION E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
- Use of log tables and calculators is not allowed
Section – A
The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.
Question 1.
If the concentration is expressed in moles per litre the unit of the rate constant for a first order reaction is:
(a) mole litre-1 sec-1
(b) mole litre-10
(c) sec-1
(d) mole-1
Answer:
(c) sec
Explanation: For first order,
n = 1
K = (mol)1-n Ln-1 S-1
So, K = (mol)1-1 L1-1 S-1
K = S-1
Question 2.
Baeyer’s reagent is which of the following?
(a) Acidified KMnO4
(b) Aqueous KMnO4
(c) Acidified K2Cr2O7
(d) Alkaline KMnO4
Answer:
(d) Alkaline KMnO4
Explanation: Baeyer’s reagent is an alkaline KMnO4 solution. This shows a redox reaction because Baeyer’s reagent is an alkaline solution of cold potassium permanganate, which is a potent oxidant. The colour of an organic material fades from purplish-pink to brown as it reacts with double or triple bonds (- C = C – or – CC -).
Question 3.
In which of the following solutions will the Van’t Hoff Factor for the solute be lesser than 1?
(a) Sodium chloride in water
(b) Benzoic acid in benzene
(c) Acetic acid in benzene
(d) Phenol in benzene
Answer:
(a) Sodium chloride in water
Explanation: The Van’t Hoff factor is less than 1 for solutes that dissociate in solution. In the given list, only sodium chloride dissociates in water whereas the remaining carboxylic acids associate in benzene. Hence, the value of the Van’t Hoff factor is lesser than 1 for a solution of sodium chloride in water.
![]()
Question 4.
Which of the following is a test for proteins?
(a) Beilstein test
(b) Biuret test
(c) Benedict’s test
(d) Molish’s test
Answer:
(b) Biuret test
Explanation: Biuret test is a test that detects the presence of chains of amino acids (proteins). If the colour changes from pale, light blue to darker blue, purple or lavender, it indicates the presence of two or more polypeptide bonds (and thus a chain of amino acids). The intensity of the dark blue/ purple colour is directly proportional to the amount of protein in the sample. A purple response will be considered +++, a light lilac, lavender or pale violet will be ++, a darker light blue is +, a slight change from light to medium blue is considered +/- and no changes (staying pale, light blue) will be negative.
Question 5.
In a plot of log K vs. 1/T, the slope is:
(a) \(\frac{-\mathrm{E}_a}{2.303}\)
(b) \(\frac{\mathrm{E}_a}{2.303 \mathrm{R}}\)
(c) \(\frac{E_a}{2.303}\)
(d) \(\frac{-\mathrm{E}_a}{2.303 \mathrm{R}}\)
Answer:
(d) \(\frac{-\mathrm{E}_a}{2.303 \mathrm{R}}\)
Explanation: Rate constant of any reaction is given by the formula,
k = Ae-Ea/RT
Taking log on both side:
log k = log A – \(\frac{\mathrm{E}_a}{2.303 \mathrm{RT}}\)
Therefore, slope of log k vs 1/T graph is given by \(\frac{\mathrm{-E}_a}{2.303 \mathrm{R}}\)
Question 6.
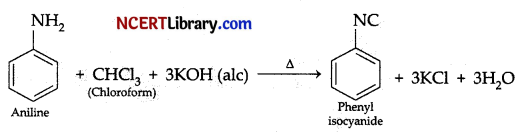
The product formed when aniline is warmed with chloroform and alcoholic KOH is:
(a) Phenyl chloride
(b) Methyl isocyanide
(c) Phenyl isocyanide
(d) Nitrophenol
Answer:
(c) Phenyl isocyanide
Explanation: When aniline reacts with chloroform and alcoholic KOH it gives an offensive smelling liquid i.e., phenyl isocyanide as product. It is called as isocyanide test. It is given by aliphatic and aromatic primary amines.
Question 7.
On oxidation with a mild oxidising agent like Br2/H2O, the glucose is oxidized to:
(a) Saccharic acid
(b) Glucaric acid
(c) Gluconic acid
(d) Valeric acid
Answer:
(c) Gluconic acid
Explanation: On oxidation with a mild oxidising agent like Br2/HzO, the glucose is oxidised to six carbon carboxylic acid (gluconic acid). This indicates that the carbonyl group is present as an aldehydic group.
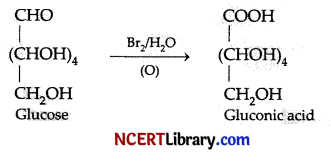
Question 8.
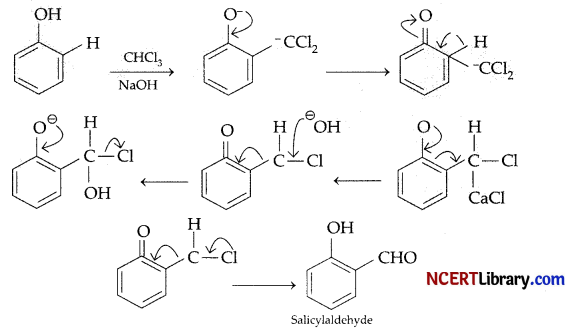
In the reaction of phenol with CHCl3 and aqueous NaOH at 70°C, the electrophile attacking the ring is:
(a) CHCl3
(b) CCl2
(c) CHCl2
(d) COCl2
Answer:
(c) CHCl2
Explanation: In the reaction of phenol with CHCl3 and aqueous NaOH at 70°C, the electrophile attacking the ring is CCl2. This is known as Reimer Tiemann Reaction.
Reimer Tiemann Reaction makes ortho substituted phenol:
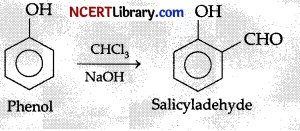
Mechanism:
Question 9.
If the rate of a reaction is expressed by, rate = A [A]² [B], the order of reaction will be:
(a) 2
(b) 3
(c) 1
(d) 0
Answer:
(b) 3
Explanation: Rate = k [A]² [B]
Order is the sum of the power of concentration of reactants.
∴ Order = 2 + 1 = 3
Hence, order of the given reaction is 3.
![]()
Question 10.
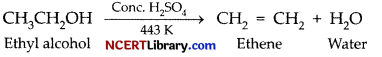
Ethanol on heating with cone. H2SO4 at 443 K gives:
(a) Diethyl sulphate
(b) Ethylene, C2H4
(c) Diethyl ether, (C2H5)2O
(d) Ethyl hydrogensulphate, C2H5HSO4
Answer:
(b) Ethylene, C2H4
Explanation: When ethanol is heated with cone. H2SO4 at 443K, the product obtained is ethylene.
Question 11.
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about:
(a) 10 times
(b) 24 times
(c) 32 times
(d) 64 times
Answer:
(b) 32 times
Explanation: For every 10°C rise of temperature, the rate is doubled. Thus, the temperature coefficient of the reaction = 2
When temperature is increased by 50°C, rate becomes
= 2(50/10)
= 25 times
= 32 times
Question 12.
The standard emf of a galvanic cell involving cell reaction with n = 2 is formed to be 0.295 V at 25° C. The equilibrium constant of the reaction would be:
(a) 1.0 x 1010
(b) 2.0 x 1011
(c) 4.0 x 1012
(d) 1.0 x 10²
[Given F = 96500 (mol-1), R = 8.314JK-1 mol-1]
Answer:
(a) 1.0 x 1010
Explanation: For a cell reaction in equilibrium at 298K.
E°cell = \(\frac { 0.0591 }{ n }\)log Kc
⇒ log Kc = \(\frac{\mathrm{E}_{\text {cell }}^{\circ} \times n}{0.0591}\)
Substitute the given values of E°cell and n in the above equation, we get
log Kc = \(\frac{0.295 \times 2}{0.0591}\)
⇒ log Kc = 10
⇒ log Kc = 1 x 1010
Question 13.
The relative order of esterification of alcohol is:
(a) 1° < 2° < 3°
(b) 1° > 2° > 3°
(c) 1° > 3° > 2°
(d) 1° < 3° < 2°
Answer:
(b) 1° > 2° > 3°
Explanation: Esterification is the process in which an organic acid (RCOOH) combines with an alcohol (ROH) to form a fruity smell product ester (RCOOR) and water. The relative order of esterification of alcohols is 1° > 2° > 3°. This is because as the steric hinderance (or bulkiness) increases from primary to secondary to tertiary alcohol, the removal of H+ will be difficult, hence, the order of esterification decreases.
Question 14.
Which of the following is a factor that automobile engines use to increase the rate of its internal reactions?
(a) Concentration of fuel
(b) Viscosity of the fuel
(c) Surface area of the reactants
(d) Nature of the fuel
Answer:
(c) Surface area of the reactants
Explanation: Automobile engines cannot change the concentration, viscosity or the nature of the fuel. In the engines, fuel is injected in the form of microscopic droplets so that it has a larger surface area than when fed into the engine as a stream. This allows the fuel to burn rapidly.
Question No. 15 to 18 consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
![]()
Question 15.
Assertion (A): Molecular mass of KC1 calculated on the basis of colligative properties will be lower than the normal molecular mass.
Reason (R): Experimentally determined molar mass is always lower than the true value.
Answer:
(c) A is true, but R is false.
Explanation: KCl undergoes dissociation in solution, hence, observed molar mass will be lower. Experimentally, determined molar mass can be higher or lower depending upon whether solute undergoes dissociation or association. Hence, assertion is true but reason is false
Question 16.
Assertion (A): Tetrahedral complexes do not show geometrical isomerism.
Reason (R): Unidentate ligands attached to the central metal ion are same.
Answer:
(a) Both A and R are true and R is the correct explanation of A.
Explanation: Tetrahedral complexes do not show geometrical isomerism because relative positions of the unidentate ligands attached to the central metal atom are same with respect to each other. Thus, both assertion and reason are correct statements and reason is the correct explanation of assertion.
Question 17.
Assertion (A): Many trivalent lanthanoid ions are coloured both in solid state and in aqueous solution.
Reason (R): Colour of these ions is due to the presence of f-electrons.
Answer:
(a) Both A and R are true and R is the correct explanation of A.
Explanation; Many trivalent lanthanoid ions are coloured both in the solid state and in aqueous solutions and colour of these ions is due to the presence of f-electrons. Thus, both assertion and reason are correct statements and reason is the correct explanation of assertion.
Question 18.
Assertion (A): Tert-butyl alcohol undergoes acid catalysed dehydration readily than propanol.
Reason (R): 3° alcohols do not give Victor-Meyer’s test.
Answer:
(b) Both A and R are true and R is not the correct explanation of A.
Explanation: Alcohol which forms the more stable carbocation undergoes dehydration more readily. Since tert-butyl alcohol forms more stable tert-butyl cation, therefore, it undergoes dehydration most readily than propanol. Primary (1°) and secondary (2°) alcohols give Victor-Meyer’s test but not tertiary (3°) alcohol. Hence, both assertion and reason are correct but reason is not the correct explanation for assertion.
Section – B
This section contains 7 questions with internal choice in two questions. The following questions are very short answer type and carry 2 marks each.
Question 19.
(a) Mention the factors that affect the rate of a chemical reaction.
(b) Why can’t molecularity of any reaction be equal to zero?
Answer:
(a) The factors that effect the rate of a reaction are as follows.
- Concentration of reactants (pressure in case of gases.)
- Temperature.
- Presence of catalyst.
(b) Molecularity of any reaction is given as the number of the reactant molecules or species that are colliding simultaneously in the elementary reaction. A minimum of one reactant is needed to initiate a chemical reaction. Therefore, the molecularity of the reaction cannot be zero.
Question 20.
(a) A solution of water and methanol is which type of mixture?
(b) What is the role of common salt in de-icing?
OR
Explain why elevation in boiling point of equimolal solution of urea and sodium chloride is different.
Answer:
(a) A solution of water and methanol is minimum boiling azeotropic mixture.
(b) De-icing is a process of removal of snow, ice and frost from surfaces such as roads using de-icing agents. De-icing agents such as common salt decreases the freezing point of ice and eventually melts the ice deposited and clears the surface.
OR
Elevation in boiling point is a colligative property and depends upon number of solute molecules in solution. Elevation in boiling point of equimolal solution of urea and sodium chloride is different due to different number of solute molecules as molecular weights are different for urea and sodium chloride.
Question 21.
(a) Which would undergo SN1 reaction faster in the following pair:

(b) What is the effect of branching on the boiling point of isomeric haloalkanes?
OR
(a) Name two types of ions formed during the reactions which undergo SN1 and SN2 reactions.
(b) What is Sandmeyer’s reaction? Give example.
Answer:
(a)

will undergo SN1 reaction faster due to stable carbocation (3°carbocation).
(b) Boiling point decreases with increasing branching.
OR
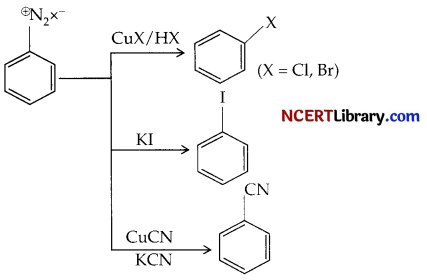
(a) Carbocation and cabanion.
(b) A reaction where diazonium salt is used for the preparation of halide is termed as sandmeyer reaction.
Question 22.
Despite having an aldehyde group, glucose does not give 2, 4-DNP test. What does this indicate? What is the significance of D and (+) here?
Answer:
Glucose does not have open chain structure and hence it does not have a free – CHO group. Actually – CHO group combines with C5 – OH to form a hemiacetal.
Clucose largely exists in the cyclic hemiacetal form along with a very small amount (0.5%) of the open chain form. Since the concentration of the open chain form is low and its reaction with 2, 4-DNP is reversible, therefore, formation of 2, 4-DNP derivative cannot disturb the equilibrium to generate more of the open chain form from the cyclic hemiacetal form and hence, it does not react with 2, 4-DNP.
The capital letter D in the name D-(+)- glucopyranose indicates that the C5 – OH group is oriented towards right while the sign (+) indicates that glucopyranose is dextrorotatory.
![]()
Question 23.
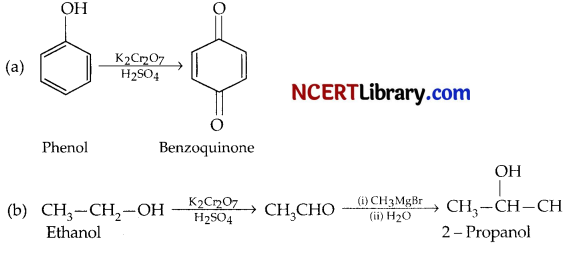
How would you convert the following:
(a) Phenol to benzoquinone.
(b) Ethanol to 2 – Propanol.
Answer:
Question 24.
(a) (CH3)2 NH is more basic than (CH3)3 N in an aqueous solution. Explain.
(b) How will you differentiate between aniline and ethylamine?
Answer:
(a) In aqueous solution 2° amine is more basic than 3° amine due to the combined effect of the inductive effect, solvation effect and steric factor.
(b) As aniline is an aromatic primary amine on with HNO2 at 0°-5°C followed by treatment with an alkaline salution of p-naphthol gives an orange coloured azo dye. But ethylamine does not give this test.
Question 25.
Amongst the following ions which one has the highest magnetic moment value?
(a) [Cr(H2O)6]3+
(b) [Fe(H2O)6]2+
(c) [Zn(H2O)6]2+
Answer:
(a) Number of unpaired electrons in [Cr(H2O)6]3+ = 3
Then, µ = Vn(n + 2)
= \(\sqrt{n(n+2)}\)
= \(\sqrt{3(n3+2)}\)
= \(\sqrt{15}\)
= ~ 4 BM
(b) Number of unpaired electrons in [Fe(H2O)6]2+ = 4
Then, µ = \(\sqrt{4(4+2)}\) = \(\sqrt{24}\) = ~ 5 BM
(c) Number of unpaired electrons in [Zn(H2O)6]2+ = 0
Hence, [Zn(H2O)6]2+ has the highest magnetic moment value, because of 4 unpaired electrons
Section – C
This section contains 5 questions with internal choice in two questions. The following questions are short answer type and carry 3 marks each.
Question 26.
(a) What are essential and non-essential amino acids? Give two examples of each.
(b) How are nucleosides different from nucleotides?
(c) Give one example each of globular protein and fibrous protein.
Answer:
(a) Non-essential amino acids: The amino acids which can be synthesised in the body, are known as non-essential amino acids. For example, Glycine, Alanine, etc.
Essential amino acids: The amino acids which cannot be synthesised in the body and must be obtained through diet are known as essential amino acids. For example, Valine, Leucine, etc.
(b) Nucleoside: A nucleoside contains only two basic components of nucleic acids i.e., a pentose sugar and a nitrogenous base. During their formation 1-position of the pyrimidine or 9-position of the purine moitey is linked to C1, of the sugar (ribose or deoxyribose) by a (ß-linkage.
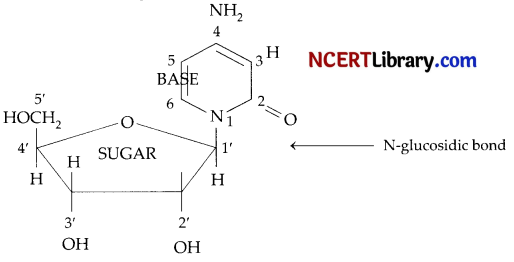
Nucleotides: A nucleotide contains all the three basic components of nucleic acids, i.e., a phosphoric acid group, a pentose sugar and nitrogenous base. These are formed by esterification of C5‘-OH of the sugar of the nucleoside with phosphoric acid.
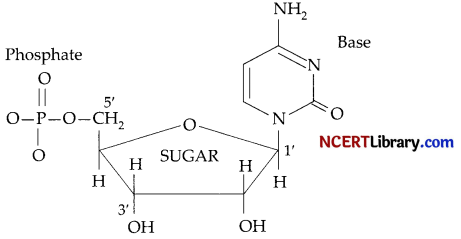
(c) Globular protein: All enzymes and hormones like insulin.
Fibrous protein: Keratin in skin, nails, etc.
Question 27.
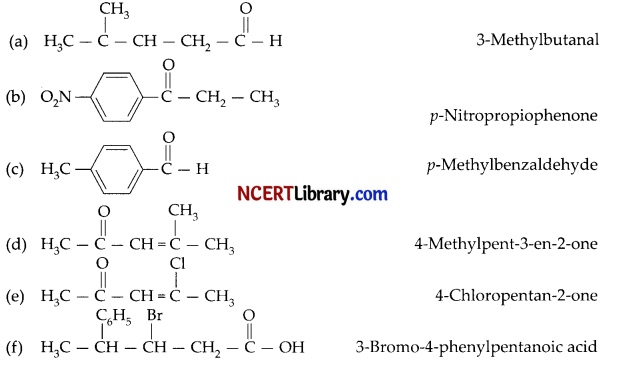
Draw the structures of the following compounds:
(a) 3-methylbutanal
(b) p-Nitropropiophenone
(c) p-Methylbenzaldehyde
(d) 4-Methylpent-3-en-2-one
(e) 4-Ch loropentan-2-one
(f) 3-Bromo-4-phenylpentanoic acid
Answer:
Question 28.
What happens when:
(a) Acetamide is treated with Br2 and NaOH.
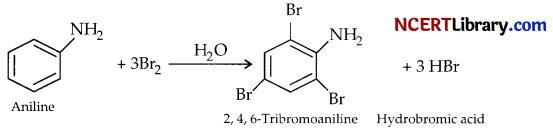
(b) Aniline is treated with bromine water.
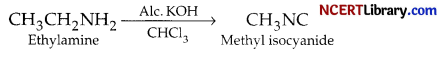
(c) Ethylamine is heated with chiorolorm and alcoholic potassium hydroxide.
Answer:
(a) Acetamide (CH3CONH2) undergoes Hoffmann Bromamide degradation in presence of bromine and NaOH to give Methanamine.
CH3CONH2 + Br2 + 4NaOH → CH3NH2 + Na2CO3 + 2NaBr + 2H2O
(b) Aniline reacts with bromine water at room temperature to give 2, 4, 6-tribromoaniline and hydrobromic acid.
(c) Ethylamine being a primary amine forms foul-smelling methyl isocyanide on heating with chloroform and alcoholic potassium hydroxide :
Question 29.
Calculate the weight of glucose which when dissolved in 100 g of water will cause lowering of vapour pressure by 0.23 mm of Hg. Vapour pressure of pure water is 54.2 mm of Hg.
Answer:
The given information is :
P1 (vapour pressure of solvent) = 54.2 mm Hg;
Molecular weight of glucose (C6H12O6), M2 = 180 g mol-1;
Lowering of vapour pressure (i.e., P1 – p1) = 0.23 mm Hg;
M1 = 18 g;
w1 = 100 g.
Relative lowering of vapour pressure can be given by the below formula :
P1 – p1 /P1 = w2 x M1/M2 x w1
Here, P1 and p1 are vapour pressure of solvent and solution respectively, w1 and w2 are masses and M1 and M2 are molar masses of the solute and solvent respectively.
Now, putting the values in equation:
0.23 mm Hg/54.2 mm Hg = w2 x 18 g mol-1/180 g mol-1 x 100 g
w2 = 4.24 g
Hence, weight of glucose is 4.24 g.
![]()
Question 30.
A solution of CuSO4 is electrolysed for 10 minutes with a current of 1.5 amperes. What is the mass of copper deposited at cathode?
OR
(a) What is the unit of specific conductance?
(b) Define the term electrochemical equivalent of an element.
(c) Name an instrument which can be used to determine electrochemical equivalent of an element.
Answer:
Given : t = 600 s; charge = current x time = 1.5 x 600 = 900 C
According to the reaction,
Cu2+ (aq) + 2e-1 → Cu (s)
We need 2 F or 2 x 96487 C to deposit 1 mol or 63 g of Cu. For 900 C, the mass of Cu deposited would be :
= \(\frac{63 \mathrm{~g} \mathrm{~mol}^{-1} \times 900 \mathrm{C}}{2 \times 96487 \mathrm{C} \mathrm{mol}^{-1}}\)
= 0.2938 g
OR
(a) Unit of specific conductance is S m-1.
(b) Electrochemical equivalent (ECE) of a substance is the amount of a substance in grams produced or consumed by the passage of one coulomb of electricity in an electrochemical reaction.
(c) A voltmeter is used to measure the electrochemical equivalent of an element.
Section – D
The following questions are case-based questions. Each question has an internal choice and carries 4 (1+1+2) marks each. Read the passage carefully and answer the questions that follow.
Question 31.
The SN² reaction mechanism involves the nucleophilic substitution reaction of the leaving group (which generally consists of halide groups or other electron-withdrawing groups) with a nucleophile in a given organic compound. The rate-determining step of this reaction depends on the interaction between the two species, namely the nucleophile and the organic compound. The SN² reaction is a good example of stereospecific reaction, one in which different stereoisomers react to give different stereoisomers of the product. Also, SN² reaction is the most common example of Walden inversion where an asymmetric carbon atom undergoes inversion of configuration.
SN² reaction were carried out for different haloalkanes and different results were obtained for them as tabulated below.
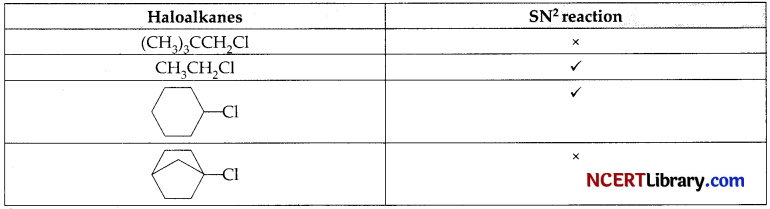
Answer the following questions:
(a) During SN² reaction which type of configuration takes place?
(b) Why CH3CH2-Cl can easily undergo SN² reaction?
(c) Why 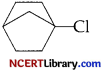 does not undergo SN² reaction?
does not undergo SN² reaction?
OR
(c) Why CH3CH2-Cl can easily undergo SN² reaction?
Answer:
(a) As during SN²2 reaction back-side approach of the nucleophile takes place, thus inversion of configuration occurs.
(b) Neopentyl halide having three alkyl groups at the p-carbon and thus, due to steric hindrance it does not undergo SN² reaction.
(c)  is a tertiary substrate and also the back side attack on a-carbon is not at all possible because of its cage like structure. So, it does not undergo SN² reaction.
is a tertiary substrate and also the back side attack on a-carbon is not at all possible because of its cage like structure. So, it does not undergo SN² reaction.
OR
CH3CH2Cl is a primary substrate and back side approach of the nucleophile is more feasible and easy. Hence SN² reaction occur here.
Question 32.
Coordination compound is any of a class of substances with chemical structures in which a central metal atom is surrounded by non-metal atoms or groups of atoms, called ligands, joined to it by chemical bonds. Coordination compounds include such substances as vitamin B12, haemoglobin, and chlorophyll, dyes and pigments, and catalysts used in preparing organic substances. A major application of coordination compounds is their use as catalysts, which serve to alter the rate of chemical reactions.
In [Fe(CN)6]4- and [Fe(H2O)6]2+, Fe is in +2 oxidation state. It is observed that dilute solutions of these complex give different colour as given in the table below:
| Metal complex | Colour |
| Dilute solution of [Fe(CN)6]4- | Light green |
| Dilute solution of [Fe(H2O)6]2+ | Pale yellow |
Answer the following questions:
(a) What is the reason for different colour?
(b) [Fe(CN)6]4- is a spin-paired on spin free complex?
(c) Explain briefly why dilute solution of [Fe(CN)6]4- gives light green colour?
OR
Explain why [Fe(H2O)6]2+ is pale yellow using crystal field splitting?
Answer:
(a) The different colours are due to the different mode of d-d electron transition.
(b) [Fe(CN)6]4- is a spin paired complex as there is no unpaired electron in the t2g level because of the strong field CNΘ ligand.
(c)

In [Fe(CN)6]4-, CNΘ is a strong field ligand and ∆0 is large and thus, it exhibits pale yellow colour.
OR
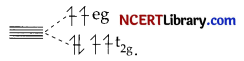
In [Fe(H2O)6]2+, H2O is weak field ligand and ∆0 is small and thus, it exhibits light green colour.
Section – E
The following questions are long answer type and carry 5 marks each. Two questions have an internal choice.
Question 33.
(a) Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points:
(i) Electronic configurations
(ii) Oxidation states
(iii) Ionisation enthalpies
(b) What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
OR
(a) What can be inferred from the magnetic moment values of the following complex species?
K4[Mn(CN)6] and magnetic moment (BM) = 2.2
[Fe(H2O)6]2+ and magnetic moment (BM) = 5.3
K2[MnCl4] and magnetic moment (BM) = 5.9
(b) How would you account for the following:
(i) Of the d4 species, Cr2+ is strongly reducing while manganese (III) is strongly oxidising.
(ii) Cobalt (II) is stable in aqueous solution but in the presence of complexing reagents, it is easily oxidised.
(c) The d1 configuration is very unstable in ions.
Answer:
(a) (i) In the 1st, 2nd and 3rd transition series, the 3d, 4d and 5d orbitals are respectively filled.
We know that elements in the same vertical column generally have similar electronic configurations.
In the first transition series, two elements show unusual electronic configurations :
Cr (24) = 3d54s1 (Because of extra stability of half filled and fully filled orbitals)
Cu (29) = 3d10 4s1
Similarly, there are exceptions in the second transition series. These are :
Mo(42) = 4d55s1 Ru(44) = 4d75s1 Pd(46) = 4d105s0
Tc(43) = 4d65s1 Rh(45) = 4d85s1 Ag(47) = 4d105s1
There are some exceptions in the third transition series as well. These are :
W(74) = 5d46s2 Pt(78) = 5d9 6s1 Au(79) = 5d106s1
As a result of these exceptions, it happens many times that the electronic configurations of the elements present in the same group are dissimilar.
(ii) In each of the three transition series, the number of oxidation states shown by the elements is the maximum in the middle and the minimum at the extreme ends.
However, +2 and +3 oxidation states are quite stable for all elements present in the first transition series. All metals present in the first transition series form stable compounds in the +2 and+3 oxidation states. The stability of the + 2 and +3 oxidation states decreases in the second and the third transition series, where in higher oxidation states are more common.
For example, chromate ion (CrO4)2- is strong oxidant while molybdate (MoO4)2- and tungstate (WO4)2- are stable.
For example: WCl6, ReF7, RuO4, etc., are stable but no such compound of 1st transition series exists.
(iii) In each of the three transition series, the first ionisation enthalpy increases from left to right. The first ionisation enthalpies of the third transition series are higher than those of the first and second transition series. This occurs due to the poor shielding effect of 4f electrons in the third transition series. The removal of one electron alters the relative energies of 4s and 3d orbitals. Hence, their is marginal and irregular increase in ionisation enthalpies.
(b) The steady decrease in atomic size with increase in atomic number among the lanthanoids on moving from left to right in a series is called lanthanoid contraction. This happens because of the increase in nuclear attraction because the addition of proton is more pronounced than the increase in the interelectronic repulsions due to the addition of electrons. The 4/electrons have poor shielding effect. Therefore, the effective nuclear charge experienced by the outer electrons increases. This results in a steady decrease in the size of lanthanoids with the increase in the atomic number.
Consequences of Lanthanoid contraction:
(i) There is similarity in the properties of second and third transition series.
(ii) Separation of lanthanoids is very difficult due to lanthanide contraction ion-exchange method is employed for that.
(iii) It is due to lanthanide contraction that covalent character increases in lanthanide hydroxides. Basic strength decreases from La(OH)3 to Lu(OH)3; where La(OH)3 is most basic and Lu(OH)3 is least basic.
OR
(a) µ is given as p = \(\sqrt{n(n+2)}\)
For value n = 1, µ = \(\sqrt{1(1+2)}\) = \(\sqrt{3}\) = 1.732
For value n = 2, µ = \(\sqrt{2(2+2)}\) = \(\sqrt{8}\) = 2.83
For value n = 3, µ = \(\sqrt{3(3+2)}\) = \(\sqrt{15}\) = 3.87
For value n = 4, µ = \(\sqrt{4(4+2)}\) = \(\sqrt{24}\) = 4.899
For value n = 5, µ = \(\sqrt{5(5+2)}\) = \(\sqrt{35}\) = 5.92
(i) K2[Mn(CN)6]
For in transition metals, the magnetic moment is calculated from the spin-only magnetic moment formula.
Therefore,
µ = \(\sqrt{n(n+2)}\) = 2.2
We can see from the above calculation that the given value is nearer to n= 1. Also, in this complex, Mn is in the +2 oxidation state. This means that Mn has 5 electrons in the d-orbital, but presence of only 1 unpaired electron suggests that CN– is a strong field ligand that causes the pairing of electrons.
(ii) [Fe(H2O|6]2+
µ = \(\sqrt{n(n+2)}\) = 5.3
We can see from the above calculation that the given value is closest to n = 4. Also, in this complex, Fe is in the +2 oxidation state. This means that Fe has 6 electrons in the d-orbital, 3d64s°, hence electron pairing has not taken place.
Hence, we can say that H2O is a weak field ligand.
(iii) K2[MnCl4]
µ = \(\sqrt{n(n+2)}\) = 5.9
We can see from the above calculation that the given value is closest to n = 5. Also, in this complex, Mn is in the + 2 oxidation state. This means that Mn has 5 electrons in the d-orbital, 3d54s°, hence pairing of electron has not occurred.
Hence, we can say that Cl’ is a weak field ligand and does not cause the pairing of electrons.
(b) (i) Cr2+ is strongly reducing in nature because of d4 configuration as a reducing agent, it gets oxidised to Cr3+ (electronic configuration, d³). This d³ configuration can be written as t2g³ configuration, which is half-filled and is more stable configuration. In the case of Mn3+ (d4), it acts as an oxidizing agent and gets reduced to Mn2+ (d5). This has an exactly half-filled d-orbital and is highly stable.
(ii) Co (II) is stable in aqueous solutions. However, in the presence of strong field complexing reagents, it is oxidised to Co (III). Although the 3rd ionisation energy for Co is high, but the . large value of crystal field stabilisation energy (CFSE) released in the presence of strong field ligands overcomes this ionisation energy.
(c) The ions in d1 configuration easily lose one more electron to get into stable d° configuration. Also, the hydration of lattice energy is more than sufficient to remove the only electron present in the d-orbital of these ions. Therefore, they act as reducing agents.
![]()
Question 34.
(a) A cell is prepared by dipping a zinc rod in 1M zinc sulphate solution and a silver electrode in 1M silver nitrate solution. The standard electrode potential given :
\(\mathrm{E}_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\circ}=-0.76 \mathrm{~V}, \mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}^{\circ}=+0.80 \mathrm{~V}\)
What is the effect of increase in concentration of Zn2+ on the Ecell?
(b) Write the products of electrolysis of aqueous solution of NaCl with platinum electrodes.
(c) Calculate e.m.f. of the following cell at 298 K :
Ni(s) | Ni2+ (0.01 M) || Cu2+ (0.1M) | Cu(s)
[Given \(\mathrm{E}_{\mathrm{Ni}^{2+} / \mathrm{Ni}}^{\circ}\) = – 0.25 V, \(\mathrm{E}^{\circ} \mathrm{Cu}^{2+} / \mathrm{Cu}\) = + 0.34 V]
Write the overall cell reaction.
OR
(a) Apply Kohlrausch law of independent migration of ions, write the expression to determine the limiting molar conductivity of calcium chloride
(b) Given are the conductivity and molar conductivity of NaCl solutions at 295 K at different concentrations:
| S. No. | Concentration [M] | Conductivity [Scm-1] | Molar conductivity [S cm2 mol-1] |
| 1. | 0.100 | 106.74 x 10-4 | 106.7 |
| 2. | 0.05 | 55.53 x 10-4 | 111.1 |
| 3. | 0.02 | 23.15 x 10-4 | 115.8 |
Compare the variation of conductivity and molar conductivity of NaCl solutions on dilution. Give reason.
(c) 0.1 M KC1 solution offered resistance of 100 ohms in a conductivity cell at 298 K. If the cell constant of the cell is 1.2 cm-1, calculate the molar conductivity of KCl solution.
Answer:
(a) Chemical equation for the given cell can be represented as :
Zn(s) + 2Ag+(aq) → Zn2+ + 2Ag(s)
And the Nernst equation can be written as :
\(\mathrm{E}_{\text {cell }}=\mathrm{E}_{\text {cell }}^{\circ}-\frac{\mathrm{RT}}{n \mathrm{~F}} \ln \frac{\left[\mathrm{Zn}^{+2}\right]}{\left[\mathrm{Ag}^{+}\right]^2}\)
With increase in Zn2+ concentration, cell potential drops from its initial value.
(b) Electrolysis of aqueous solution of NaCl yields NaOH, Cl2 and H2.
The net reaction can be shown as :
NaCl(aq) + H2O(1) → Na+(aq) + OH– (aq) + 1/2H2(g) + 1/2Cl2(g)
(c) The half cell reactions are :
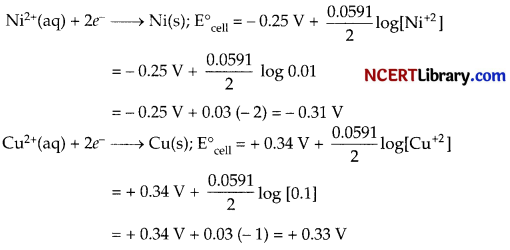
So, the overall cell reaction is :
Ni(s) + Cu2+(aq) → Ni2+(aq)+ Cu(s);
Now emf of the cell could be given by :
E° = 0.33 V – (0.31 V) = 0.64 V
OR
(a) Kohlrausch law of independent migration of ions states that limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and cation of the electrolyte. Thus if λ°Ca2+ and λ°Ca– are limiting molar conductivity of the calcium and chloride ions respectively, then the limiting molar conductivity for calcium chloride is given by the equation:
Λ°m(CaCl2) = λ°Ca2+ + 2λCa–
(b) Conductivity always decreases with the dilution, so as the case with given NaCl solution here, This can be explained by the fact that the number of ions per unit volume that carry the current in a solution decreases on dilution.
Molar conductivity of a solution at a given concentration is the conductance of the volume V of the solution containing one mole of electrolyte in a standard volume increases with decrease in concentration, same is the case here with NaCl solution. This is because the total volume, V, of solution containing one mole of electrolyte also increases.
(c) Molar conductivity= Lm = k/c
Where k is conductivity of the solution.
Now, Cell constant = G* = Conductivity x resistance
or Conductivity = Cell constant/resistance
k = 1.29 cm-1100 Ω
= 1.29 x 10-2 S cm-1
Molar conductivity = Λm = 1.29 x 10-2 S cm-1 x 1000 cm³ L-1 molarity-1
= 1.29 x 10-2 S cm-1 x 1000 cm³ L-1/0.1 mol L-1
= 1.29 S cm² mol-1.
Question 35.
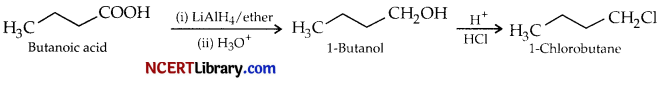
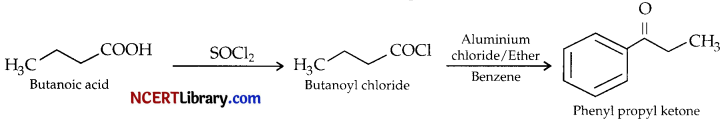
(a) Give reaction sequences for conversion of butanoic acid to :
(i) 1-chlorobutane.
(ii) Phenyl propyl ketone.
(b) Which acid would you expect to be stronger? Why?
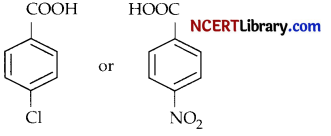
Answer:
(a) (i) Butanoic acid is first reduced to 1-butanol using lithium aluminium hydride as reducing agent, then 1-butanol undergoes nucleophilic substitution with hydrochloric acid (aq) to give 1-chlorobutane.
(ii) Butanoic acid is firstly converted to butanoyl chloride using thionyl chloride (SOCl2), which is then used for Friedal Craft’s acylation of benzene to provide Phenyl propyl ketone.
(iii) p-N itrobenzoic acid is a stronger acid than p-Chlorobenzoic acid due to its better electron withdrawing effect. Though chlorine atom withdraws electron due to its – I effect but at the same time takes part in resonance with the phenyl group through its lone pair of electrons making it less acidic than p-N itrobenzoi c acid.