Ethanol
Ethanol is an alcohol and is obtained by the fermentation of molasses which are obtained from sugarcane juice. Its molecular formula is C2H5OH. Alcohols are carbon compounds containing – OH group attached to a carbon atom. It can also be considered as derived from an alkane by replacing a hydrogen atom with a hydroxyl group (OH).
Physical Properties of Ethanol
- Ethanol is a liquid at room temperature.
- Ethanol is commonly called alcohol and is the active ingredient of all alcoholic drinks.
- It is used in medicines such as tincture iodine, cough syrups, and many tonics because it is a good solvent.
- Ethanol is also solubLe in water in all proportions.
- Long-term consumption of alcohol leads to many health problems.
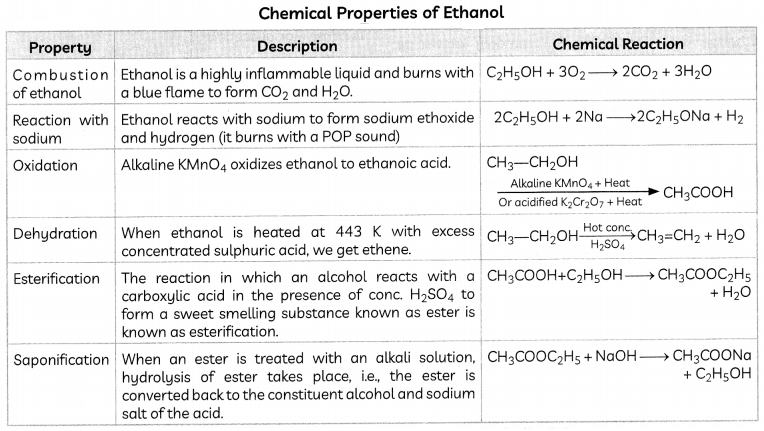
Chemical Properties of Ethanol
Effect of Alcohol on Living Beings:
- When large quantities of ethanoL are consumed, it tends to slow metabolic processes and to depress the central nervous system.
- This results in lack of coordination, mental confusion, drowsiness, lowering of the normal inhibitions, and finally stupor.
- The individual may feel relaxed but does not realise that his sense of judgement, sense of timing, and muscular coordination have been seriously impaired.
Effect of Methanol
- Intake of methanol in very small quantities can cause death.
- Methanol is oxidised to methanal in the liver.
- Methanol reacts rapidly with the components of cells. It causes the protoplasm to get coagulated, in much the same way an egg is coagulated by cooking.
- Methanol also affects the optic nerve, causing blindness.
Uses of Ethanol
- It is one of the most important organic chemicals and is used as a solvent for lacquers, varnishes, perfumes medicines, etc.
- It is used for sterilizing wounds as it is a good antiseptic.
- It is used as a fuel in internal combustion engines and as a substitute for petrol in motor cars under the name ‘power alcohol’.
- It is used for making antifreeze mixtures, which are used in the radiators of motor vehicles in cold countries.
Tests for Alcoholic Group:
- Sodium metal test: When a small piece of sodium metal is added to the organic liquid, bubbles of hydrogen gas are produced, indicating the presence of the alcoholic group.
- Ester test: When the organic liquid to be tested is warmed with some glacial acetic acid and a few drops of cone. H2SO4, a sweet-smelling substance is formed.
Denatured Alcohol
Ethanol is an important industrial chemical and is subjected to very small excise duty. To prevent its misuse for drinking purposes, the alcohol supplied for industrial, purposes is rendered unfit for drinking by mixing it with some poisonous substances, such as methanol, pyridine, copper sulphate etc. It is known as denatured alcohol.
Rectified spirit: Ethanol containing 5 per cent water is known as rectified spirit.
Harmful effects of drinking alcohol:
- Alcohol is an intoxicant. The person loses all senses of discrimination under its influence.
- If a person drinks alcohol regularly, he/she becomes dependent on it and becomes an addict.
- The body loses its control and gradually one loses one’s consciousness if the dose of alcohol is increased.
- It may even cause death if consumed in large quantities as it damages the liver.
- It worsens the economic condition of a family.
- It has a very bad effect on the psychological development of children.
Ethanoic Acid
Ethanoic acid (CH3COOH) is commonly known as acetic acid. Its dilute solution in water (5-8%) is known as vinegar and is used for preserving food. The melting point of pure ethanoic acid is 290 Kand hence it often freezes during winter in cold climates. This gave rise to its name glacial acetic acid.
Physical Properties of Ethanoic Acid
- It is a colourless, pungent-smelling liquid having a boiling point of 391 K.
- It is miscible with water in all proportions.
Chemical Properties of Ethanoic Acid

Uses of Ethanoic Acid
- It is used for making synthetic vinegar which is used for preserving food.
- It is used as a reagent in a laboratory.
- It is used for making white lead [2PbCO3. Pb(OH)2] which is used as white paint.
Example 1.
How would you distinguish experimentally between alcohol and o carboxylic acid?
Answer:
Alcohol and o carboxylic acid can be distinguished experimentally by reaction with sodium carbonate (Na2CO3) or sodium hydrogen carbonate (NaHCO3) as alcohol does not react with NaHCO3 whereas carboxylic acid reacts with NaHC03 and gives a salt, water and brisk effervescence of carbon dioxide gas.
Example: CH3COOH + NaHCO3 → CH3COONa + CO2 + H2O
Saponification Reaction
Esters are sweet-smelling substances, These are used in making perfumes and as flavouring agents. Esters react in the presence of an acid or a base to give back the alcohol and carboxylic acid. This reaction is known as saponification because it is used in the preparation of soap.