NCERT Exemplar Class 9 Science Chapter 3 Atoms and molecules are part of NCERT Exemplar Class 9 Science. Here we have given NCERT Exemplar Class 9 Science Solutions Chapter 3 Atoms and Molecules.
NCERT Exemplar Class 9 Science Solutions Chapter 3 Atoms and Molecules
Multiple Choice Questions
Question 1.
Which of the following correctly represents 3# 360 g of water?
(i) 2 moles of H20
(ii) 20 moles of water
(iii) 6.022 x 1023 molecules of water
(iv) 1.2044 x102S molecules of water
(a) (i)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Solution:
(d)
(i) 1 molt of water = 18 g 2 moles of water = 2 x 18 g = 36 g
(ii) 20 moles of water = 18 x 20 = 360 g
(iii) 6.022 x 1023 molecules of water
= 1 mole = 18 g
(iv) 1 mole of water = 6.022 x 1023 molecules
6.22 x 1023 molecules = 1 mole
1.2044 x 1025molecules =
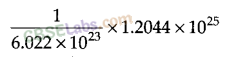
= 20 moles = 20 x 18 = 360 g
Question 2.
Which of the following statements is not true about an atom?
(a) Atoms are not able to exist independently.
(b) Atoms are the basic units from which molecules and ions are formed.
(c) Atoms are always neutral in nature.
(d) Atoms aggregate in large numbers to form the matter that we can see, feel or touch.
Solution:
(a) Atoms of inert gases exist in monoatomic or independent form.
Question 3.
The chemical symbol for nitrogen gas is
(a) Ni
(b) N2
(c)N+
(d) N
Solution:
(b) Nitrogen gas exists as a diatomic molecule hence, its symbol is N2.
Question 4.
The chemical symbol for sodium is
(a) So
(b) Sd
(c) NA
(d) Na
Solution:
(d) Chemical symbol for sodium is Na.
Question 5.
Which of the following would weigh the highest?
(a) 0.2 mole of sucrose (C12H22O11)
(b) 2 moles of CO2
(c) 2 moles of CaCO3
(d) 10 moles of H2O
Solution:
(c)Weight = Numberpf moles x molar mass.
0.2 mole of sucrose (C12H22O11) = 0.2 x 342
= 68.4 g
2 moles of CO2 = 2 x 44 = 88 g
2 moles of CaCO3 = 2 x 100 = 200 g
10 moles of H2O = 10 x 18 = 180 g
Question 6.
Which of the following has maximum number of atoms?
(a) 18g of H2O
(b) 18g of O2
(c) 18g of CO2
(d) 18 g of CH4
Solution:
(d) Number of atoms =

Question 7.
Which of the following contains maximum number of molecules?
(a) 1g CO2 (b) 1g N2
(c) 1g H2 (d) 1g CH4
Solution:
(c) ag of CO2=1/44 x 6.022 x 1023 molecules
= 1.368 x 1022molecules

Question 8.
Mass of one atom of oxygen is
(a)\(\frac{16}{6.023 \times 10^{23}} \mathrm{g}\)
(b)\(\frac{23}{6.023 \times 10^{23}} \mathrm{g}\)
(c)\(\frac{1}{6.023 \times 10^{23}} \mathrm{g}\)
(d)8 u
Solution:
Mass of one atom of oxygen
=Atomic mass/N
\(=\frac{16}{6.022 \times 10^{23}} \mathrm{g}\)
Question 9.
3.42 g of sucrose are dissolved in 18 g of water in a beaker. The number of oxygen atoms in the solution are
(a) 6.68 x1023
(b) 6.09 x1022
(c) 6.022 x 1023
(d) 6.022.x 1021
Solution:
(a) Number of moles of sucrose
3.42/342=0.01mol–

Question 10.
A change in the physical state can be brought about
(a) only when energy is given to the system
(b) only when energy is taken out from the system
(c) when energy is either given to, or taken out from the system
(d) without any energy change.
Solution:
(c) When energy is given to the system the solid state changes to liquid. When energy is taken out from a liquid it changes to solid e.g., ice changes to water and water to water vapours when heat energy is given. Water vapours or steam condense to water and water freezes to ice when energy is decreased.
Short Answer Type Questions
Question 11.
Which of the following represents a correct chemical formula? Name it.
(a) CaCl
(b) BiPO4
(c) NaSO4
(d) NaS
Solution:
(a) The correct formula is CaCl2 (valency of Ca = 2, valency of Cl = 1).
(b) is correct because valency of Bi = 3, valency of PO4 = 3
(c) The correct formula is Na2S04 (valency of Na = 1, Valency of S04 = 2).
The correct formula is Na2 (valency of Na = 1, valency of sulphide = 2).
Thus, the correct formula is BiP04 and the name is bismuth phosphate.
Question 12.
Write the molecular formulae for the following compounds:
(a) Copper (II) bromide
(b) Aluminium (III) nitrate
(c) Calcium (II) phosphate
(d) Iron (III) sulphide
(e) Mercury (II) chloride
(f) Magnesium (II) acetate.
Solution:
(a) Copper(II) Bromide – cuBr2
(b) Aluminium(III) nitrate – A1(N03)3
(c) Calciuih(II) phosphate – Ca3(PO4)2
(d) Iron(III) sulphide – Fe2S3
(e) Mercury(II) chloride – HgCl2
(f) Magnesium(II) acetate – Mg(CH3COO)2
Question 13.
Write the molecular formulae of all the compounds that can be formed by the combination of following ions:
\(\mathrm{Cu}^{2+}, \mathrm{Na}^{+}, \mathrm{Fe}^{3+}, \mathrm{Cl}^{-}, \mathrm{SO}_{4}^{2-}, \mathrm{PO}_{4}^{3-}\)
Solution:
\(\begin{array}{l}{\mathrm{Cl}^{-}, \mathrm{SO}_{4}^{2-}, \mathrm{PO}_{4}^{3-} \text { anions }} \\ {\mathrm{CuCl}_{2}, \mathrm{CuSO}_{4}, \mathrm{Cu}_{3}\left(\mathrm{PO}_{4}\right)_{2}} \\ {\mathrm{NaCl}, \mathrm{Na}_{2} \mathrm{SO}_{4}, \mathrm{Na}_{3} \mathrm{PO}_{4}} \\ {\mathrm{FeCl}_{3} \mathrm{Fe}_{2}\left(\mathrm{SO}_{4}\right)_{3}, \mathrm{FePO}_{4}} \\ {\text { Write the cations and anions }}\end{array}\)
Question 14.
Write the cations and anions present (if any) in the following compounds:
(a) CH3COONa
(b) NaCl
(c) H3
(d) NH4NO3
Solution:
\(\begin{array}{l}{\text { (b) } \mathrm{NaCl}-\mathrm{Na}^{+}, \mathrm{Cl}^{-}} \\ {\text { (c) } \mathrm{H}_{2}-\text { It is a covalent compound hence, no }} \\ {\text { ions are present in it. }} \\ {\text { (d) } \mathrm{NH}_{4} \mathrm{NO}_{3}-\mathrm{NH}_{4}^{+}, \mathrm{NO}_{3}^{-}}\end{array}\)
Question 15.
Give the formulae of the compounds formed from the following sets of elements:
(a) Calcium and fluorine
(b) Hydrogen and sulphur
(c) Nitrogen and hydrogen
(d) Carbon and chlorine
(e) Sodium and oxygen
(f) Carbon and oxygen
Solution:
(a) Calcium fluoride – CaF2
(b) Hydrogen sulphide – H2S
(c) Ammonia – NH3
(d) Carbon tetrachloride – CCl4
(e) Sodium oxide – Na20
(f) Carbon monoxide – CO
(g(Carbon dioxide – C02
Question 16.
Which of the following symbols of elements are incorrect? Give their correct symbols.
(a) Cobalt – CO
(b) Carbon – c
(c) Aluminium – AL
(d) Helium – He
(e) Sodium – So
Solution:
(a) Cobalt-Co
(b) Carbon – C
(c) Aluminium-A1
(d) Helium – He (correct)
(e) Sodium-Na
Question 17.
Give the chemical formulae for the following compounds and compute the ratio by mass of the combining elements in each one of them. You may use appendix -111
(a) Ammonia
(b) Carbon monoxide
(c) Hydrogen chloride
(d) Aluminium fluoride
(e) Magnesium sulphide.
Solution:
| Chemical formula | Ratio by mass | |
| (a) | Ammonia (NH3) | N(l): H(3) 14:3 |
| (b) | Carbon monoxide (CO) | 0(1): 0(1)12 :16 or 3 : 4 |
| (c) | Hydrogen chloride (HC1) | H(l): Cl(l)1 : 35.5 or 2 : 71 |
| (d) | Aluminium fluoride (AlF3) | Al(l): F(3)27 :19 x 3 or 9 :19 |
| (e) | Magnesium sulphide (MgS) | Mg(l): S(l)24 : 32 or 3 :4 |
Question 18.
State the number of atoms present in each of the following chemical species:
(a) CO32-
(b) PQ43-
(c) P205
(d) CO
Solution:
\(\begin{array}{l}{\text { (a) } \mathrm{CO}_{3}^{2-}=1 \mathrm{C}+3(\mathrm{O})=4} \\ {\text { (b) } \mathrm{PO}_{4}^{3-}=1 \mathrm{P}+4(\mathrm{O})=5} \\ {\text { (c) } \mathrm{P}_{2} \mathrm{O}_{5}=2 \mathrm{P}+5(\mathrm{O})=7} \\ {\text { (d) } \mathrm{CO}=1 \mathrm{C}+1(\mathrm{O})=2}\end{array}\)
Question 19.
What is the fraction of the mass of water due to neutrons?

Question 20.
Does the solubility of a substance change with temperature? Explain with the help of an example.
Solution:
Yes, solubility of a substance changes with temperature. It generally increases with temperature. More sugar can be dissolved in hot water as compared to cold water.
Question 21.
Classify each of the following on the basis of their atomicity:

Solution:
Monoatomic with atomicity (1) = He, Ag

Question 22.
You are provided with a fine white coloured powder which is either sugar or salt. How would you identify it without tasting?
Solution:
On heating, sugar powder is charred and becomes black while salt does not char. When dissolved in water, salt solution will conduct electricity because it is ionic while sugar solution will not conduct electricity because it is covalent.
Question 23.
Calculate the number of moles of magnesium present in a magnesium ribbon weighing 12 g. Molar atomic mass of magnesium is 24 g mol-1. Solution:
Atomic mass of Mg = 24 g mol-1
24 g of Mg = 1 mol
12 g of Mg = 12/24 = 0.5 mol
Long Answer Type Questions
Question 24.
Verify by calculating that
(a) 5 moles of CO2 and 5 moles of H2O do not have the same mass.
(b) 240 g of calcium and 240 g magnesium elements have a mole ratio of 3:5.
Solution:
(a) Mass of one mole CO2=44g

Question 25.
Find the ratio by mass of the combining elements in the following compounds. (You may use appendix -111)



Question 26.
Calcium chloride, when dissolved in water, dissociates into its ions according to the following equation:


Question 27.
The difference in the mass of 100 moles each of sodium atoms and sodium ions is 0.0548002 g. Compute the mass of an electron.
Solution:
Number of electrons in Na atom = 11
Number of electrons in Na+ = 10
For 1 mole of Na atom and Na+ the difference in electrons = 1 mole
For 100 moles of Na atoms and Na ions the
difference = 100 moles of electrons
Mass of 100 moles of electrons = 0.0548002 g

Question 28.
Cinnabar (HgS) is a prominent ore of mercury. How many grams of mercury are present in 225 g of pure HgS? Molar mass of Hg and S are 200.6 g mol-1 and 32 g mol-1 respectively.
Solution:
Molar mass of HgS = 200.6 + 32 = 232.6 g
Mass of Hg in 232.6 g of HgS = 200.6 g

Question 29.
The mass of one steel screw is 4.11g. Find the mass of one mole of these steel screws. Compare this value with the mass of the Earth (5.98 x 1024 kg). Which one of the two is heavier and by how many times?
Solution:
Mass of 21 screw = 4.11 g

Question 30.
A sample of vitamin C is known to contain 2.58 x1024 oxygen atoms. How many moles of oxygen atoms are present in the sample?
Solution:
Number of oxygen atoms in the sample=2.58 x 1024

Question 31.
Raunak took 5 moles of carbon atoms in a container and krish also took 5 moles of sodium atoms in another container of same weighty.
(a) Whose container is heavier?
(b) Whose container has more number of atoms?
Solution:
(a) Mass of container containing 5 moles of C atoms=5 x 12 = 60 g
Mass of caontaner containing 5 moles of Na atoms=5 x 23 = 115 g
Hence, container of Krish is heavier.
(b) Both containers have same number of atoms since they co0ntailn same number of moles.
Question 32.
Fill in the missing data in the Table 3.1
| SpeciesProperty | h2o | C02 | Na atom | MgCI2 |
| No. of moles | 2 | – | 0.5 | |
| No. of particles | – | 3.01 lx 1023 | – | |
| Mass | 36 g | – | 115 g | – |
Solution:
| Species Property | h2o | co2 | Na atom | MgCl2 |
| No. of moles | 2 | 0.5 | 5 | 0.5 |
| No. of particles | 1.2044 x 1024 | 3.011 x 1023
|
5×6.022 x1023 = 3.011 x 1024 | 0.5 x6.022 x 1023 x 3 = 9.033 x 1023 |
| Mass | 36 g | 22 g | 115 g | 47.5 g |
Question 33.
The visible universe is estimated to contain 1022 stars. How many moles of stars are present in the visible universe?
Solution:

Question 34.
What is the SI prefix for each of the following multiples and submultiples of a unit?

Solution:
(a) 103 = kilo

Question 35.
Express each of the following in kilograms
(a) 5.84 x 10-3mg
(b) 58.34 g
(c) 0.584 g
(d) 5.873 x 10- 21g
Solution:
(a) 5.84 x 10-3mg


Question 36.
Compute the difference in masses of 103 moles each of magnesium atoms and magnesium ions.(Mass of an electron=9.1 x 10-31 kg
Solution:
Mg+2ion= 10 electron

Question 37.
Which has more number of atoms? 100fg of N2 or 100 g of NH3
Solution:
(i) 100 g of N2 =100/28moles

Question 38.
Compute the number of ions present in 5.85 g of sodium chloride.
Solution:
1 mole of NaCl =23+35.5=58.5 g of NaCl
Number of moles in 5085 g of NaCl
=5085/58.5=0.1 moles
Each NaCl formula unit=Na++ Cl–
=2 ions

Question 39
A gold sample contains 90% of gold and the rest copper. How many atoms of gold are present in one gram of this sample of gold?
Solution:
1 g of gold sample contains

Question 40.
What are ionic and molecular compounds? Give examples.
Maim Ionic compounds are made up of ions. An ionic compound contains a cation which is a positive ion and an anion which is a negative ion e.g., sodium chloride is an ionic compound made up of Na+ and Cl– ions.
A molecular compound is made up of molecules e.g. ammonia (NH3), carbon dioxide (CO2).
Question 41.
Compute the difference in masses of one mole each of aluminium atoms and one mole of its ions. (Mass of an electron is 9.1 x 10-28 g). Which one is heavier?
Solution:

Difference = 27 – 26.9984 = 0.0016 g
1 mole of Al atoms is heavier than 1 mole of Al3+ions.
Question 42.
A silver ornament of mass ‘m’ gram is polished with gold equivalent to 1% of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament.
Solution:
Mass of gold = mg

Question 43.
A sample of ethane (C2H6) gas has the same mass as 1.5 x 102° molecules of methane (CH4). How many C2H6molecules does the sample of gas contain?
Solution:
Molar mass of CH4=12+4=16 g mol-1

Question 44.
(a) In a chemical reaction, the sum of the masses of the reactants and products remains unchanged. This is called _____.
(b) A group of atoms carrying a fixed charge on them is called _____.
(c) The formula unit mass of Ca3(P04)2 is _____.
(d) Formula of sodium carbonate is _____. and that of ammonium sulphate is _____.
Solution:
(a) Law of conservation of mass
(b) Polyatomic ion
(c) 310

Question 45.
Complete the following crossword puzzle (Fig. 3.1) by using the name of the chemical elements. Use the data given in Table 3.2 Table 3.2
| Across | Down | ||
| 2. | The element used by Rutherford during his a-scattering experiment. | 1. | A white lustrous metal used for making ornaments and which tends to get tarnished black in the presence of moist air. |
| 3. | An element which forms rust on exposure to moist air. | 4. | Both brass and bronze are alloys of the element. |
| 5. | A very reactive non-metal stored under water. | 6. | The metal which exists in the liquid state at room temperature. |
| 7. | Zinc metal when treated with dilute hydrochloric acid produces a gas of this element which when tested with burning splinter produces a pop sound. | 8. | An element with symbol Pb. |

Solution:

Question 46.
a) In this crossword puzzle (Fig. 3.2), names of 11 elements are hidden. Symbols of these are given below. Complete the puzzle.
1. Cl
2. H
3. Ar
4. 0
5. Xe
6. N
7. He
8. F
9. Kr
10. Rn
11. Ne

(b) Identify the total number of inert gases, their names and symbols from this crossword puzzle.
Solution:

Question 47.
Write the formulae f8r the following and
calculate the molecular mass for each one of them:
(a) Caustic potash
(b) Baking powder
(c) Lime stone
(d) Caustic soda
(e) Ethanol
(f) Common salt
Solution:
(a) caustic potash, KOH
=(39+16+1)=56gmol-


Question 48.
In photosynthesis, 6 molecules of carbon dioxide combine with an equal number of water molecules through a complex series of reactions to give a molecule of glucose having a molecular formula C6H1206. How many grams of water would be required to produce 18 g of glucose? Compute the volume of water so consumed assuming the density of water to be 1 g cm-3.
Solution:
![]()

NCERT Exemplar Class 9 Science Solutions
- Chapter 1 Matter In Our Surroundings
- Chapter 2 Is Matter Around Us Pure
- Chapter 3 Atoms and Molecules
- Chapter 4 Structure of the Atom
- Chapter 5 The Fundamental Unit of Life
- Chapter 6 Tissues
- Chapter 7 Diversity in Living Organisms
- Chapter 8 Motion
- Chapter 9 Force and Laws of Motion
- Chapter 10 Gravitation
- Chapter 11 Work and Energy
- Chapter 12 Sound
- Chapter 13 Why do we Fall ill
- Chapter 14 Natural Resources
- Chapter 15 Improvement in Food Resources
We hope the NCERT Exemplar Class 9 Science Chapter 3 Atoms and Molecules will help you. If you have any query regarding NCERT Exemplar Class 9 Science Solutions Chapter 3 Atoms and Molecules, drop a comment below and we will get back to you at the earliest.