Important Questions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers Class 12 Important Questions
Alcohols, Phenols and Ethers Class 12 Important Questions Very Short Answer Type
Question 1.
Give the IUPAC name of the following compound : (Delhi 2009)
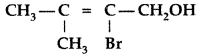
Answer:

IUPAC name : 2-Bromo-3-methyl-but-2-ene-1-ol
Question 2.
Give the IUPAC name of the following (All India 2009)

Answer:

Question 3.
Write the structure of the molecule of a compound whose IUPAC name is 1-phenylpropan-2-ol. (All India 2010)
Answer:
1-phenylpropan-2-ol

Question 4.
How would you convert ethanol to ethene? (All India 2011)
Answer:

Question 5.
Draw the structure of 2, 6-Dimethylphenol. (All India 2011)
Answer:

Question 6.
Draw the structural formula of 2-methylpropan- 2-ol molecule. (Delhi 2012)
Answer:

Question 7.
Draw the structure of hex-l-en-3-ol compound. (Delhi 2012)
Answer:
CH2 = CH – CH(OH) – CH2 – CH2 – CH3
Question 8.
Ortho nitrophenol has lower boiling point than p-nitrophenol. Why ? (Comptt. Delhi 2012)
Answer:
Ortho-nitrophenol has lower boiling point due to formation of intramolecular H-bonding whereas p-nitrophenol forms intermoleeular H-bonding.
Question 9.
Ortho-nitrophenol is more acidic than ortho-methoxyphenol. Why? (Comptt. Delhi 2012)
Answer:
NO2 group is an electron withdrawing group while methoxy group is electron donating in nature. The release of H+ is easier from O-nitrophenol while it is difficult from O-methoxyphenol.
Question 10.
The C-O bond is much shorter in phenol than in ethanol. Give reason. (Comptt. Delhi 2012)
Answer:
Carbon of C-O bond of phenol is Sp2 hybridised, so it acquires a partial double bond character but in ethanol it is Sp3 hybridised and a single bond. Double bond is shorter than a single bond.
Question 11.
Write the IUPAC name of the following : (Comptt. All India 2012)

Answer:
IUPAC name : 2-Bromo-3-methyl but-2-en-l-ol.
Question 12.
Of the two hydroxy organic compounds ROH and R’OH, the first one is basic and other is acidic in behaviour. How is R different from R’? (Comptt. Delhi 2013)
Answer:
When R = alkyl, ROH behaves as a bronsted base and when R’ = aryl, R’OH behaves as a bronsted acid.
Question 13.
Give a chemical test to distinguish between 2-Pentanol and 3-Pentanol. (Comptt. Delhi 2013)
Answer:
2-pentanol gives Iodoform test with yellow ppt. of Iodoform while 3-pentanol does not give this test.
Question 14.
Write the chemical reaction to explain Kolbe’s reaction. (Comptt. Delhi 2013)
Answer:
Kolbe’s reaction : Phenol reacts with CO2 in presence of sodium hydroxide (NaOH) at 4 – 7 Atm and 390 – 410 K giving salicylic acid

Question 15.
How would you obtain ethane-1, 2-diol from ethanol? (Comptt. All India 2013)
Answer:

Question 16.
How would you obtain acetophenone from phenol? (Comptt. All India 2013)
Answer:

Question 17.
Write IUPAC name of the following : (Comptt. All India 2013)

Answer:
IUPAC name : 2~Bromo-3-methylbut -2-ene-l-ol
Question 18.
How would you obtain phenol from benzene? (Comptt. All India 2013)
Answer:

Question 19.
Write IUPAC name of the following (Comptt. All India 2013)

Answer:
IUPAC name : 2-Methoxy-5-methyl phenol
Question 20.
Which of the following isomers is more volatile : o-nitrophenol or p-nitrophenol? (Delhi 2014)
Answer:
o-nitrophenol is more volatile than p-nitrophenol due to intramolecular hydrogen bonding.
Question 21.
Name the alcohol that is used to make the following ester : (Comptt All India 2014)

Answer:
Alcohol used : Propan-2-ol
Question 22.
Write the IUPAC name of the given compound (Delhi 2015)

Answer:
2, 5-dinitrophenol.
Question 23.
Write the IUPAC name of given compound: (All India 2015)

Answer:
IUPAC name : 1-Ethoxy-2-methylpropane
Question 24.
Write the IUPAC name of the given compound: (All India 2016)

Answer:
2-Phenylethanol
Question 25.
Write equation of the nitration of anisole. (Comptt. Delhi 2016)
Answer:

Question 26.
Out of CH3OH and
 , which one is more acidic? (Comptt. All India 2016)
, which one is more acidic? (Comptt. All India 2016)
Answer:
 is more acidic, as Phenoxide formed is more stabilized by Resonance.
is more acidic, as Phenoxide formed is more stabilized by Resonance.
Question 27.
Write the IUPAC name of the following compound: (All India 2017)

Answer:
2-Bromo-3-methylbut-2-enol-1 -ol
Question 28.
Write the IUPAC name of the following compound: (All India 2017)

Answer:
3-phenylprop-2-en-1-ol
Question 29.
Write IUPAC name of the following compound : (Comptt. Delhi 2017)

Answer:
2, 3-Dinitro phenol.
Question 30.
What happens when phenol is oxidized by Na2Cr2O7/H2SO4?
Answer:
Phenol forms benzoquinone on oxidation with Na2Cr2O7 / H2SO4,

Question 31.
What happens when phenol is heated with zinc dust? (Comptt. All India 2017)
Answer:
Benzene is formed when phenol is heated with zinc dust.

Question 32.
What happens when phenol is treated with bromine water? (Comptt. All India 2017)
Answer:
2, 4, 6-tribromophenol is formed when phenol is treated with bromine water.

Question 33.
Give simple chemical tests to distinguish between the following pairs of compounds: Benzoic acid and Phenol (All India 2017)
Answer:
Ferric chloride test. Add neutral FeCl3 in both the solutions, phenol reacts with neutral FeCl3 to form an iron-phenol complex giving violet colour but benzoic acid does not.
Alcohols, Phenols and Ethers Class 12 Important Questions Short Answer Type -I [SA – I]
Question 34.
Complete the following reaction equations : (Delhi 2009)

Answer:

Question 35.
Illustrate the following reactions giving a chemical equation for each :
(i) Kolbe’s reaction
(ii) Williamsons synthesis of an ether (Delhi 2010)
Answer:
(i) Kolbe’s reaction : Phenol reacts with CO2 in presence of sodium hydroxide (NaOH) at 4 – 7 Atm and 390 – 410 K giving salicylic acid

(ii) Williamsons synthesis of an ether : The reaction involves the nucleophilic substitution of the halide ion from the alkyl halide by the alkoxide ion by SN2 mechanism.
Example :

Question 36.
Explain the following reactions with an example for each :
(i) Reimer-Tiemann reaction
(ii) Friedel-Crafts reaction. (Delhi 2010)
Answer:
(i) Reimer-Tiemann reaction : Treatment of phenol with CHCl3 in presence of aqueous NaOH at 340K followed by hydrolysis gives salicylaldehyde.

(ii) Friedel-Crafts reaction : This reaction is used for introducing an alkyl or an acyl group into an aromatic compound in presence of Lewis acid catalyst (AlCl3)
Example:

Question 37.
How are the following conversions carried out?
(i) Propene to propan-2-ol
(ii) Ethylmagnesium chloride to propan-1-ol. (Delhi 2010
Answer:
(i) Propene to propan-2-ol

(ii) Ethylmagnesium chloride to propan-1-ol

Question 38.
How are the following conversions carried out?
(i) Benzyl chloride to benzyl alcohol,
(ii) Methyl magnesium bromide to 2-methylpropan-2-ol. (All India 2010)
Answer:
(i) Benzyl chloride to benzyl alcohol

Question 39.
Explain the following giving one example for each :
(i) Reimer-Tiemann reaction.
(ii) Friedel-Craft’s acetylation of anisole. (Delhi 2011)
Answer:
(i) Reimer-Tiemann reaction : Treatment of phenol with CHCl3 in presence of aqueous NaOH at 340K followed by hydrolysis gives salicylaldehyde.

(ii) Friedel-Craft’s acetylation of anisole :

Question 40.
How would you obtain
(i) Picric acid (2, 4, 6-trinitrophenol) from phenol,
(ii) 2-Methylpropene from 2-methylpropanol? (Delhi 2011)
Answer:
(i) Picric acid from phenol :

Question 41.
Explain the mechanism of acid catalysed hydration of an alkene to form corresponding alcohol. (All India 2012)
Answer:
Acid catalysed hydration : Alkenes react with water in the presence of acid as catalyst to form alcohols

Mechanism : It involves three steps :
(i) Protonation of alkene to form carbocation by electrophilic attack of H3O+

Question 42.
Explain the following behaviours :
(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.
(ii) Ortho-nitrophenol is more acidic than ortho-methoxyphenol. (All India 2012)
Answer:
(i) Alcohols can form H-bonds with water and break the H-bonds already existing between water molecules. So they are soluble in water.

On the other hand, hydrocarbons cannot form H-bonds with water and hence are insoluble in water.
(ii) Due to strong – R and -1 effect of the – NO2 group, electron density in the – OH bond decreases and hence the loss of a proton becomes easier. Moreover O-nitrophenoxide ion is stabilized by resonance, 1 thereby making O-nitrophenol a stronger acid.
In O-methoxyphenol, due to + R effect of the – OCH3 group the electron density in the O – H bond increases thereby making the loss of proton difficult. Furthermore, the Q-methoxyphenoxide ion left after the loss of a proton is destabilized by resonance because the two negative charges repel each other. So O-methoxyphenol is a weaker acid.
Question 43.
Explain the mechanism of following reaction: (Delhi 2013)
![]()
Answer:

Question 44.
How will you convert:
(i) Propene to propan-2-ol?
(ii) Phenol to 2, 4, 6-trinitrophenol? (Delhi 2013)
Answer:
(i) Propene to propan-2-ol:

(ii) Phenol to 2, 4, 6-trinitrophenol?

Question 45.
How will you convert the following?
(i) Propan-2-ol to propanone
(ii) Phenol to 2, 4, 6-tribromophenol (Delhi 2013)
Answer:
(i) Propan-2-ol to propane

(ii) Phenol to 2, 4, 6-tribromophenol: Phenol reacts with bromine in presence of polar solvent H2O to form 2, 4, 6-tribromophenol (white ppt.)

Question 46.
How will you convert:
(i) Propene to Propane-1-ol?
(ii) Ehtanal to Propan-2-ol (Delhi 2013)
Answer:
(i) Propene to Propane-1-ol

Question 47.
Explain the mechanism of the following reaction: (All India 2013)
![]()
Answer:

Question 48.
Write the equations involved in the following reactions:
(i) Reimer-Tiemann reaction
(ii) Williamson’s ether Synthesis (All India 2013)
Answer:
(i) Reimer-Tiemann reaction : Treatment of phenol with CHCl3 in presence of aqueous NaOH at 340K followed by hydrolysis gives salicylaldehyde.

(ii) Williamson’s ether synthesis : The reaction involves the nucleophilic substitution of the halide ion from the alkyl halide by the alkoxide ion by SN2 mechanism.
Example :

Question 49.
Write the equations involved in the following reactions :
(i) Reimer – Tiemann reaction
(ii) Williamson synthesis (All India 2014)
Answer:
(i) Reimer-Tiemann reaction : Treatment of phenol with CHCl3 in presence of aqueous NaOH at 340K followed by hydrolysis gives salicylaldehyde.

(ii) Williamson’s ether synthesis : The reaction involves the nucleophilic substitution of the halide ion from the alkyl halide by the alkoxide ion by SN2 mechanism.
Example :

Question 50.
Write the mechanism of the following reaction : (All India 2014)
![]()
Answer:


Question 51.
Write the equations involved in the following reactions:
(i) Williamson ether synthesis
(ii) Kolbe’s reaction (Comptt. Delhi 2014)
Answer:
(i) Williamson ether synthesis : The reaction involves the nucleophilic substitution of the halide ion from the alkyl halide by the alkoxide ion by SN2 mechanism.
Example :

(ii) Kolbe’s reaction : Phenoxide ion generated by treating phenol with sodium hydroxide is more reactive than phenol and undergoes electrophilic substitution with carbon dioxide. Ortho hydroxybenzoic acid is formed as the main reaction product.

Question 52.
How are the following conversions carried out?
(i) Propene to Propan-2-ol
(ii) Ethyl chloride to Ethanal (Comptt. Delhi 2014)
Answer:
(i) Propene to propan-2-ol

Question 53.
Explain the mechanism of dehydration steps of ethanol : (Comptt. Delhi 2015)
![]()
Answer:

Question 54.
Write the mechanism of acid dehydration of ethanol to yield ethene. (Comptt. All India 2015)
Answer:
The mechanism of dehydration of ethanol involves the following steps :
Step 1 : Formation of protonated alcohol


Question 55.
Write the mechanism of the following reaction: (Delhi 2016)
![]()
Answer:
Mechanism:

Question 56.
(a) Arrange the following compounds in the increasing order of their acid strength: p-cresol, p-nitrophenol, phenol
(b) Write the mechanism (using curved arrow notation) of the following reaction: (All India 2016)
![]()
Answer:
(a) Order of acidic strength:
p-cresol < phenol < p-nitrophenol
(b) Mechanism of acid catalysed hydration of alkene:
Step 1: Protonation of alkene to form carbocation by electrophilic attack of H3O+.

Question 57.
Write the structures of the products when Butan-2-ol reacts with the following:
(a) CrO3
(b) SOCl2 (All India 2016)
Answer:

Alcohols, Phenols and Ethers Class 12 Important Questions Short Answer Type -II [SA – II]
Question 58.
Explain the mechanism of the following reactions :
(i) Addition of Grignard’s reagent to the carbonyl group of a compound forming an adduct followed by hydrolysis.
(ii) Acid catalysed dehydration of an alcohol forming an alkene.
(iii) Acid catalysed hydration of an alkene forming an alcohol. (Delhi 2009)
Answer:
(i) Carbonyl group undergoes nucleophillic addition reaction with Grignard reagent to form an adduct which undergoes hydrolysis to give alcohol in the following manner:

(ii) The mechanism of dehydration of ethanol involves the following steps :
Mechanism : It involves the following three steps :
Step 1 : Formation of protonated alcohol

(iii) Acid catalysed hydration : Alkenes react with water in the presence of acid as catalyst to form alcohols. Mechanism : It involves the following three steps :
Step 1 : Protonation of alkene to form carbocation by electrophilic attack of H3O+


Question 59.
Explain the following observations :
(i) The boiling point of ethanol is higher than that of methoxymethane.
(ii) Phenol is more acidic than ethanol.
(iii) o- and p-nitrophenols are more acidic than phenol. (All India 2009)
Answer:
(i) Due to presence of intermolecular H-bonding, associated molecules are formed, hence ethanol has high boiling point while methoxymethane does not have intermolecular H-bonding.
(ii) Phenol on losing H+ ion forms phenoxide ion, and ethanol on losing H+ ion forms ethoxide ion. Phenoxide ion is more stable than ethoxide ion as phenoxide ion exists in resonance structure. Due to this phenol is more acidic than ethanol.
(iii) Both o- and p-nitrophenols contain the NO2 group which is an electron withdrawing group. Due to -R and -I effect of the -NO2 group, electron density in the OH bond of substituted phenol decreases and hence the loss of proton becomes easy and therefore more acidic.
Question 60.
How would you convert the following :
(i) Phenol to benzoquinone
(ii) Propanone to 2-methylpropan-2-ol
(iii) Propene to propan-2-ol (All India 2010)
Answer:
(i) Phenol to benzoquinone

Question 61.
How would you obtain the following ;
(i) Benzoquinone from phenol
(ii) 2-Methylpropan-2-ol from ethylmagnesium chloride
(iii) Propan-2-ol from propene (All India 2010)
Answer:
(i) Benzoquinone from phenol

(ii) Ethylmagnesium chloride to propan-1-ol

(iii) Propene to propan-2-ol

Question 62.
Draw the structure and name the product formed if the following alcohols are oxidized. Assume that an excess of oxidising agent is used.
(i) CH3CH2CH2CH2OH
(ii) 2-butanol
(iii) 2-methyl-l-propanol (Delhi 2012)
Answer:

Question 63.
(a) Illustrate the following name reactions :
(i) Reimer-Tiemann Reaction (ii) Williamson Synthesis.
(b) Give a chemical test to distinguish between 2-propanol and 2-methyl-2-propanol. (Comptt. Delhi 2012)
Answer:
(a) (i) Reimer-Tiemann reaction : Treatment of phenol with CHCl3 in presence of aqueous NaOH at 340K followed by hydrolysis gives salicylaldehyde.

(ii) Williamson’s ether synthesis : The reaction involves the nucleophilic substitution of the halide ion from the alkyl halide by the alkoxide ion by SN2 mechanism.
Example :

(b) 2-Propanol is a secondary alcohol. When it reacts with I2 in NaOH, it forms a yellow ppt of iodoform but 2-methyl-2 propanol does not respond to this test.
Question 64.
(a) Give a seperate chemical test to distinguish between the following pairs of compounds:
(i) Ethanol and Phenol (ii) 2-Pentanol and 3-Pentanol
(b) Explain Kolbe’s reaction with the help of suitable example. (Comptt. Delhi 2012)
Answer:
(a) (i) Ethanol on reacting with I2 in NaOH gives yellow ppt of iodoform whereas phenol does not respond to this test.
(ii) 2-Pentanol on reacting with I2 in NaOH gives yellow ppt of iodoform whereas 3-pentanol does not respond to this test.
(b) Kolbe’s reaction : Phenol reacts with NaOH to give sodium phenoxide which on reaction with CO2 in acid gives salicylic acid.

Question 65.
(a) How would you obtain the following :
(i) 2-methylpentan-2-ol from 2-methyl-1-pentene
(ii) Acetophenone from phenol
(b) Write IUPAC name of the following : (Comptt. All India 2012)

Answer:
(a) (i) 2-Methylpentan-2-ol from 2-methyl-1-pentene

(b) IUPAC name : 1 -ethoxy-2-nitrocyclohexane.
Question 66.
(a) Give mechanism of preparation of ethoxy ethane from ethanol.
(b) How is toluene obtained from phenol? (Comptt. Delhi 2013)
Answer:
(a) Mechanism of formation of ethoxy ethane
Step 1 : Protonation of ethanol

Question 67.
(a) Give chemical tests to distinguish between the following pairs of compounds :
(i) Pentan-2-ol and Pentan-3-ol (ii) Methanol and Phenol
(b) o-nitro phenol is more acidic than o-methoxy phenol. Explain why. (Comptt. All India 2013)
Answer:
(a) (i) 2-pentanol gives Iodoform test with yellow ppt. of Iodoform while 3-pentanone does not give this test.
(ii) Distinction between Methanol and Phenol :
By FeCl3 test : Phenol gives violet coloured solution with FeCl3 while methanol does not.

(b) O-nitro phenol is more acidic than o-methoxy phenol due to presence of NO2 group which has -I effect. It weakens the O-H bond of phenol by withdrawing their electrons and thus releases H+ ion easily while due to +I, +R effect of OCH3, o-methoxy phenol is less acidic.
Question 68.
(a) Write the mechanism of the following reaction :
![]()
(b) Write the equation involved in Reimer-Tiemann reaction. (Delhi 2014)
Answer:


(b) Ortho-nitrophenol has lower boiling point due to formation of intramolecular H-bonding whereas p-nitrophenol forms intermoleeular H-bonding.
Question 69.
Explain the following with an example for each :
(i) Kolbe’s reaction
(ii) Reimer-Tiemann reaction
(iii) Williamson ether synthesis (Comptt. All India 2014)
Answer:
(i) Kolbe’s reaction: Phenoxide ion generated by treating phenol with sodium hydroxide is more reactive than phenol and undergoes electrophilic substitution with carbon dioxide. Ortho hydroxybenzoic acid is formed as the main reaction product.

(ii) Reimer-Tiemann reaction : Treatment of phenol with CHCl3 in presence of aqueous NaOH at 340K followed by hydrolysis gives salicylaldehyde.

(iii) Williamson’s ether synthesis : The reaction involves the nucleophilic substitution of the halide ion from the alkyl halide by the alkoxide ion by SN2 mechanism.
Example :

Question 70.
How are the following conversions carried out?
(i) Propene → 4 Propan-2-ol
(ii) Ethylmagnesium chloride → 4 Propan-l-ol
(iii) Benzyl chloride → Benzyl alcohol (Comptt All India 2014)
Answer:
(i) Propene to propan-2-ol

(ii) Ethylmagnesium chloride to propan-1-ol

(iii) Benzyl chloride → Benzyl alcohol

Question 71.
How do you convert the following :
(i) Phenol to anisole
(ii) Propan-2-ol to 2-methylpropan-2-ol
(iii) Aniline to phenol (Delhi 2015)
Answer:

Question 72.
(a) Write the mechanism of the following reaction :
![]()
(b) Write the equation involved in the acetylation of Salicylic acid (Delhi 2015)
Answer:

Question 73.
Give reasons for the following :
(i) Phenol is more acidic than methanol.
(ii) The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle (190°28′).
(iii) (CH3)3C—O—CH3 on reaction with HI gives (CH3)3C—I and CH3—OH as the main products and not (CH3)3C—OH and CH3—I. (All India 2015)
Answer:
(i) Phenol is more acidic than methanol because in phenol, phenoxide ion formed is more stabilized by resonance than phenol. There is no resonance in methanol.
(ii) The C—O—H bond angle in alcohols is slightly less than tetrahedral angle due to repulsion between the lone pairs of electrons of oxygen.
(iii) (CH3)3C+ is 3° carbo-cation which is more stable than \(\mathrm{CH}_{3}^{+}\) for SN1 reaction.
Question 74.
How are the following conversions carried out?
(i) Propene to propan-2-ol
(ii) Benzyl chloride to Benzyl alcohol
(iii) Anisole to p-Bromoanisole (Comptt. Delhi 2015)
Answer:
(i) Propene to propan-2-ol

Question 75.
Write the major product in the following equations : (Comptt. All India 2015)

Answer:


Question 76.
Write the main product(s) in each of the following reactions: (Delhi 2016)

Answer:


Question 77.
Write the final product(s) in each of the following reactions: (All India 2016)

Answer:

Question 78.
How are the following conversions carried out?
(i) Propene → Propan-2-ol
(ii) Benzyl chloride → Benzyl alcohol
(iii) Ethyl magnesium chloride → Propan-1-ol (Comptt. Delhi 2016)
Answer:

Question 79.
Explain the following with an example in each :
(i) Kolbe’s reaction
(ii) Reimer-Tiemann reaction
(iii) Williamson ether synthesis (Comptt. All India 2016)
Answer:
(i) Kolbe’s reaction : Phenol reacts with CO2 at 390-400 K under pressure 4-7 giving Selicyle Acid


Question 80.
(a) What happens when CH3—O—CH<sub3 is heated with HI?
(b) Explain mechanism for hydration of acid catalyzed ethene :
CH2 = CH2 + HzO \(\stackrel{\mathrm{H}^{+}}{\longrightarrow} \)CH3—CH,—OH (Comptt. Delhi 2017)
Answer:
(a) Methyl Iodide (CH3I) and Methanol (CH3OH) are formed when CH3—O—CH3 is heated with HI.
![]()
(b) Acid catalysed hydration : Alkenes react with water in the presence of acid as catalyst to form alcohols.
Mechanism : It involves the following three steps :
Step 1: Protonation of alkene to form carbocation by electrophilic attack of H3O+.

Question 81.
(a) Why phenol is more acidic than ethanol?
(b) Write the mechanism of acid dehydration of ethanol to yield ether: (Comptt. All India 2017)![]()
Answer:
(a) Phenol on losing H+ ion forms phenoxide ion, and ethanol on losing H+ ion forms ethoxide ion. Phenoxide ion is more stable than ethoxide ion as phenoxide ion exists in resonance structure. Due to this phenol is more acidic than ethanol.


Question 82.
(i) Write Reimer-Timann reaction.
(ii) Write the mechanism of acid dehydration of ethanol to yield ethene : (Comptt. All India 2017)![]()
Answer:
(i) Reimer-Timman reaction—

Alcohols, Phenols and Ethers Class 12 Important Questions long Answer Type (LA)
Question 83.
(a) Write the product(s) in the following reactions: (Delhi 2017)

(b) Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Ethanol and Phenol
(ii) Propanol and 2-methylpropan-2-ol
Answer:


Question 84.
Write the formula of reagents used in the following reactions:
(i) Bromination of phenol to 2,4,6-tribromophenol
(ii) Hydroboration of propene and then oxidation to propanol.
(b) Arrange the following compound groups in the increasing order of their property indicated:
(i) p-nitrophenol, ethanol, phenol (acidic character)
(ii) Propanol, Propane, Propanal (boiling point)
(c) Write the mechanism (using curved arrow notation) of the following reaction: (Delhi 2017)

Answer:
