Practicing the CBSE Sample Papers for Class 12 Chemistry with Solutions Set 1 allows you to get rid of exam fear and be confident to appear for the exam.
CBSE Sample Papers for Class 12 Chemistry Set 1 with Solutions
Time : 3 Hours
Maximum Marks: 70
General Instructions :
Read the following instructions carefully.
- There are 35 questions in this question paper with internal choice.
- SECTION A consists of 18 multiple-choice questions carrying 1 mark each.
- SECTION B consists of 7 very short answer questions carrying 2 marks each.
- SECTION C consists of 5 short answer questions carrying 3 marks each.
- SECTION D consists of 2 case-based questions carrying 4 marks each.
- SECTION E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
- Use of log tables and calculators is not allowed
Section – A
The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.
Question 1.
The major product of acid catalysed dehydration of 1-methylcyclohexanol is: [1]
(a) 1-methylcyclohexane
(b) 1-methylcyclohexene
(c) 1-cyclohexylmethanol
(d) 1-methylenecyclohexane
Answer:
(b) 1-methylcyclohexene
Explanation: Acid catalysed dehydration of 1-methylcyclohexanol reaction:
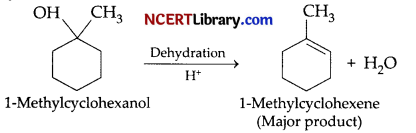
The above reaction follows saytzeff’s rule i.e., highly substituted alkene is major product.
Since, here we have 3° alcohol, on dehydration, 1-methylcyclohexene would be the major product by the removal of a water molecule.
Question 2.
Which one of the following compounds is more reactive towards SN1 reaction? [1]
(a) CH2 = CHCH2Br
(b) C6H5CH2Br
(c) C6H5CH(C6H5)Br
(d) C6H5CH(CH3)Br
Answer:
(c) C6H5CH(C6H5)Br
Explanation: For haloalkanes containing same functional group, the order of reactivity of SN1 reaction depends upon stability of Carbocation intermediate. CH2 = CHCH2Br and C6H5CH2Br will form primary carbocation intermediate.
Of the two secondary bromides, the carbocation intermediate obtained from C6H5CH(C6H5)Br is more stable than obtained from C6H5CH(CH3)Br because it is stabilised by two phenyl groups due to resonance. Therefore, the former bromide is more reactive than the latter in SNl reactions.
![]()
Question 3.
KMnO4 is coloured due to: [1]
(a) d-d transitions
(b) charge transfer from ligand to metal
(c) unpaired electrons in d-orbital of Mn
(d) charge transfer from metal to ligand
Answer:
(b) charge transfer from ligand to metal
Explanation: In KMnO4 Oxidation state of Manganese = +7
Electronic configuration = [Ar]3d04s0
Due to the absence of unpaired electrons, d-d transition in KMnO4 molecule is not possible. Thus, the molecule should be colourless. Therefore, the intense purple colour of potassium permanganate is due to the charge transfer from ligand to metal, 2p(L) of O to 3d(M) of Mn.
Question 4.
Which radioactive isotope would have the longer half- life 15O or 19O? (Given rate constants for 15O and 19O are 5.63 x 10-3s-1 and k = 2.38 x 10-2s-1 respectively). [1]
(a) 15O
(b) 19O
(c) Both will have the same half-life
(d) None of these
Answer:
(a) 15O
Explanation: The radioactive decay reaction follows the first order mechanics. Therefore, for the given value of rate constant, the half life will be given by:
λ = \(\frac{0.693}{T_{1 / 2}}\)
Therefore, the half life of a radioactive element is inversely proportional to rate constant. The rate constant for the decay of 15O is less than that for 15O. Therefore , the rate of decay of 15O will be slower and will have a longer half life.
Question 5.
The molar conductivity of CH3COOH at infinite dilution is 390 S cm²/mol. Using the graph and given information, the molar conductivity of CH3COOK will be: [1]

(a) 100 S cm²/mol
(b) 115 S cm²/mol
(c) 150 S cm²/mol
(d) 125 S cm²/mol
Answer:
(b) 115 S cm²/mol
Explanation: According to Kohlrausch’s law at infinite dilution, Molar conductivity of weak electrolyte CH3COOH will be,
A°CH3COOK = A°CH3COOH + A°KCl – A°HCl
= 390 + 150 – 425
= 115 S cm²/mol
Question 6.
For the reaction, A + 2B → AB2 the order w.r.t. reactant A is 2 and w.r.t. reactant B. What will be change in rate of reaction if the concentration of A is doubled and B is halved? [1]
(a) increases four times
(b) decreases four times
(c) increases two times
(d) no change
Answer:
(a) increases four times
Explanation: For the reaction, A + 2B → AB2
The order of reaction w.r.t. A is 2 and w.r.t. B is 0.
Hence, Rate = [A]²
If the concentration of A is doubled, A’ = 2A
Rate’ = [A’]² = [2A]² = 4 [A]²
Question 7.
Arrange the following in the increasing order of their boiling points: [1]
A : Butanamine, B: N,N-Dimethylethanamine, C: N- Ethylethanamine.
(a) C < B < A
(b) A < B < C
(c) A < C < B
(d) B < C < A
Answer:
(d) B < C < A
Explanation: Amines have a tendency to form hydrogen bonds with each other. Hence, amines have high boiling point. Since, in primary amine, intermolecular association due to H-bonding is maximum while in tertiary amine, it is minimum therefore, the boiling point will be highest for primary amine and lowest for tertiary amines.
Question 8.
The CFSE of [CoC6]3- is 18000 cm-1. The CFSE for [CoCl4]– will be: [1]
(a) 18000 cm-1
(b) 8000 cm-1
(c) 2000 cm-1
(d) 16000 cm-1
Answer:
(b) 8000 cm-1
Explanation: According to crystal field theory,
∆t = 4/9 ∆0
Therefore, for the given complex,
∆t = 4/9 x 18000 cm-1
= 8000 cm-1
![]()
Question 9.
What would be the major product of the following reaction? [1]
C6H5 – CH2 – OC6H5 + HBr → A + B
(a) A = C6H5CH2OH, B = C6H6
(b) A = C6H5CH2OH, B = C6H5Br
(C) A = C6H5CH2OH, B = C6H5Br
(d) A = C6H5CH2Br, B = C6H5OH
Answer:
(d) A = C6H5CH2Br, B = C6H5OH
Explanation: In the case of alkyl aryl ethers, the compound is cleaved at the alkyl-oxygen bond due to the more stable aryl-oxygen bond. The reaction yields phenol and alkyl halide.
Therefore, for the given compound:
C6H5 – CH2 – OC6H5 + HBr → C6H5 – CH2 – Br + C6H5OH
Question 10.
Which of the following statements is not correct for amines? [1]
(a) Most alkyl amines are more basic than ammonia solution.
(b) pKb value of ethylamine is lower than benzylamine.
(c) CH3NH2 on reaction with nitrous acid releases NO2 gas.
(d) Hinsberg’s reagent reacts with secondary amines to form sulphonamides.
Answer:
(c) CH3NH2 on reaction with nitrous acid releases NO2 gas.
Explanation:
Option (a) is the correct statement because due to the presence of electron donating alkyl groups, most alkyl amines are more basic than ammonia solution.
Option (b) is the correct statement because due to resonance, the lone pair of electrons are not available in benzyl amine. Therefore, pKb value of ethylamine is lower than benzylamine.
Optidn (c) is the incorrect statement because when primary amines reacts with nitrous add, the evolution of Nitrogen gas takes place not NOz gas.
Option (d) is the correct statement because when Hinsberg’s reagent reacts with secondary amines, sulphonamides are formed which is insoluble in an alkali.
Question 11.
Which of the following tests/ reactions is given by aldehydes as well as ketones? [1]
(a) Fehling’s test
(b) Tollen’s test
(c) 2, 4 DNP test
(d) Cannizzaro reaction
Answer:
(c) 2, 4 DNP test
Explanation: 2,4-Dinitrophenyl hydrazine (DNP) test is the test that is given by both aldehydes and ketones. While Fehling’s, Tollen’s and Cannizzaro reactions are shown by aldehydes only.
Question 12.
Arrhenius equation can be represented graphically as follows:

The (i) intercept and (ii) slope of the graph are:
(a) (i) In A (ii) Ea/R
(b) (i) A (ii) Ea
(c) (i) In A (ii) – Ea/R
(d) (i) A (ii) – Ea
Answer:
(c) (i) In A (ii) – Ea/R
Explanation: Arrhenius equation is, k = Ae-Ea/RT
Taking natural logarithm of both sides of equation In k = – Ea/RT + In A
According to the given equation, The plot of 1n k vs 1/T gives a straight line where slope = – Ea/R and intercept = 1n A.
Question 13.
The number of ions formed on dissolving one molecule of FeSO4.(NH4)2SO4.6H2O in water is: [1]
(a) 3
(b) 4
(c) 5
(d) 6
Answer:
(c) 5
Explanation: On dissolving one molecule of given double salt (FeSO4.(NH4)2SO4.6H2O) in water, the ions formed are Fe+2, 2NH+4, 2SO42-.
Question 14.
The oxidation of toluene to benzaldehyde by chromyl chloride is called: [1]
(a) Etard reaction
(b) Riemer-Tiemann reaction
(c) Stephen’s reaction
(d) Cannizzaro’s reaction
Answer:
(a) Etard reaction
Explanation: Chromyl chloride oxidises methyl group to a chromium complex, which on hydrolysis gives corresponding benzaldehyde. This reaction is called Etard reaction.

Question No. 15 to 18 consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true and R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Question 15.
Assertion (A): An ether is more volatile than an alcohol of comparable molecular mass. [1]
Reason (R): Ethers are polar in nature.
Answer: (b) Both A and R are true and R is not the correct explanation of A.
Explanation: An ether has a higher volatility than an alcohol with the same molecular formula. This is because inter molecular hydrogen bonding occurs in alcohols. It raises the boiling point of alcohol while decreasing its volatility. Others, such as ethers, do not allow for such inter-molecular hydrogen bonding. Thus, both A and R are true but R is not the correct explanation of A.
![]()
Question 16.
Assertion (A): Proteins are found to have two different types of secondary structures viz. alpha-helix and beta-pleated sheet structure. [1]
Reason (R): The secondary structure of proteins is stabilized by hydrogen bonding.
Answer:
(b) Both A and R are true and R is not the correct explanation of A.
Explanation: The – NH group of each amino acid residue is hydrogen-bonded to the C = O of an adjacent turn of helix. In the a-helix, the most common ways a polypeptide chain exists and forms all possible hydrogen bonds by twisting into a right-handed screw (helix). Thus, both A and R are true but R is not the correct explanation of A.
Question 17.
Assertion (A): Magnetic moment values of actinides are lesser than the theoretically predicted values.
Reason (R): Actinide elements are strongly paramagnetic. [1]
Answer:
(b) Both A and R are true and R is not the correct explanation of A.
Explanation: Due to the presence of large number of unpaired electrons, actinides are paramagnetic in nature.
Moreover, Magnetic moment values of actinides are lesser than the theoretically predicted values. This is because 5f electrons of actinides are less effectively shielded which result in quenching of orbital contribution. Thus, both A and R are true but R is not the correct explanation of A.
Question 18.
Assertion (A): Tertiary amines are more basic than corresponding secondary and primary amines in gaseous state. [1]
Reason (R): Tertiary amines have three alkyl groups which cause +1 effect.
Answer:
(a) Both A and R are true and R is the correct explanation of A.
Explanation: The presence of electron donating alkyl group makes the electron density on the alkylamine’s nitrogen (+1 effect increases) greater than the nitrogen of ammonium.
Tertiary amines have three alkyl groups which cause +1 effect due to which basic characters are more in it. Thus, both A and R are true and R is the correct explanation of A.
Section – B
This section contains 7 questions with internal choice in two questions. The following questions are very short answer type and carry 2 marks each.
Question 19.
A first-order reaction takes 69.3 min for 50% completion. What is the time needed for 80% of the reaction to get completed?
(Given: log 5 = 0.6990, log 8 = 0.9030, log 2 = 0.3010) [2]
Answer:
Given, A → Product

Question 20.
Account for the following:
(a) There are 5 OH groups in glucose
(b) Glucose is a reducing sugar
OR
What happens when D – glucose is treated with the following reagents
(a) Bromine water
(b) HNO3
Answer:
(a) Acetylation of glucose with acetic anhydride gives glucose pentaacetate which confirms the presence of five -OH groups. Since it exists as a stable compound, five -OH groups should be attached to different carbon atoms.

(b) Carbohydrates which reduce Fehling’s solution and Tollens’ reagent are reducing sugars. When glucose reacts with Fehling’s reagent, it get oxidised to Gluconic acid.

OR
(a) D-glucose gets oxidised to six carbon carboxylic acid (gluconic acid) on reaction with a mild oxidising agent like bromine water. This indicates that the carbonyl group is present as an aldehydic group.

(b) On oxidation with nitric acid, D-glucose as well as gluconic acid both yield a dicarboxylic acid, saccharic acid. This indicates the presence of a primary alcoholic (-OH) group in glucose.

Question 21.
Give reason for the following: [2]
(a) During the electrophilic substitution reaction of haloarenes, para substituted derivative is the major product.
(b) The product formed during SNa reaction is a racemic mixture.
OR
(a) Name the suitable alcohol and reagent, from which 2-Chloro-2-methyl propane can be prepared.
(b) Out of the Chloromethane and Fluoromethane, which one is has higher dipole moment and why?
Answer:
(a) When electrophilic substitution reaction takes place, then ortho and para products are formed among them para is considered as the major product and ortho as a minor product because of the steric hindrance. In the ortho product, the groups are present next to each other this causes the hindrance and the group tries to attach at the para position. Hence para product predominates over ortho product.
(b) A mixture containing two enantiomers in equal proportions will have zero optical rotation, as the rotation due to one isomer will be cancelled by the rotation due to the other isomer. Such a mixture is known as racemic mixture.
During the SN1 mechanism, intermediate carbocation formed is sp² hybridized and is planar in nature. This allows the attack of nucleophile from either side of the plane resulting in the racemic mixture.
OR
(a) Formation of 2-Chloro-2-methyl propane
Reagent used – Lucas reagent (Cone. HCl + ZnCl2)

(b) The dipole moment is based on the product of distance and charge, rather than just charge alone, CH3Cl has a larger dipole moment than CH3F.
Although fluorine is more electronegative than chlorine, the carbon-fluorine bond is also much shorter than carbon-chlorine bond: 139 pm vs 178 pm. As a result, the dipole moment of chloromethane is greater than that of fluoromethane.
Question 22.
The formula Co(NH3)5CO3Cl could represent a carbonate or a chloride. Write the structures and names
of possible isomers. [2]
Answer:
The ionisation isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become the counter ion.
The given compound forms ionisation isomer, One is chloride and other one is carbonate.
[Co(NH3)5CO3]Cl Pentaaminecarbonatocobalt(III)chloride
and [Co(NH3)5Cl]CO3 Pentaaminechloridocobalt (III) carbonate
Question 23.
Corrosion is an electrochemical phenomenon. The oxygen in moist air reacts as follows: [2]
O2(g) + 2H2O(l) + 4e– → 40H–(aq)
Write down the possible reactions for corrosion of zinc occurring at anode, cathode, and overall reaction to form a white layer of zinc hydroxide.
Answer:
Corrosion is a natural process in which a refined metal is converted to a more chemically stable form, such as oxide, hydroxide, or sulphide.
Corrosion of zinc:
Anode: Zn(s) → Zn2+(aq) + 2e–
Cathode: O2(g) + 2H2O(l) + 4e– → 40H–(aq.)
Overall: 2Zn (s) + O2(g) + 2H2O(l) → 2Zn2+(aq.) + 4OH–(aq.)
![]()
Question 24.
Explain how and why will the rate of reaction for a given reaction be affected when [2]
(a) a catalyst is added.
(b) the temperature at which the reaction was taking place is decreased.
Answer:
(a) A catalyst participates in a chemical reaction by forming temporary bonds with the reactants resulting in an intermediate complex. This has a transitory existence and decomposes to yield products and the catalyst.
The catalyst provides an alternate pathway or reaction mechanism by reducing the activation energy between reactants and products and hence lowering the potential energy barrier. Therefore, the rate of reaction will increase on addition of a catalyst.
(b) For a chemical reaction with rise in temperature by 10°C, the rate constant is nearly doubled. At lower temperatures the kinetic energy of molecules decreases thereby the collisions decrease resulting in a lowering of rate of reaction.
Question 25.
Write the reaction and IUPAC name of the product formed when 2-Methylpropanal (isobutyraldehyde) is treated with ethyl magnesium bromide followed by hydrolysis. [2]
Answer:
When aldehydes and ketones are treated with grignard reagent, it undergoes nucleophilic addition reaction to produce corresponding alcohols.
When 2-Methylpropanal reacts with ethyl magnesium bromide:

2-Methylpentan-3-ol(secondary alcohol) is formed as a product.
Section – C
This section contains 5 questions with internal choice in two questions. The following questions are short answer type and carry 3 marks each.
Question 26.
Write the equations for the following reaction: [3]
(a) Salicylic acid is treated with acetic anhydride in the presence of cone. H2SO4
(b) Tert butyl chloride is treated with sodium ethoxide.
(c) Phenol is treated with chloroform in the presence of NaOH
Answer:
(a) When salicylic acid is treated with acetic anhydride in the presence of conc.H2SO4, acetylation reaction takes place and acetylsalicylic acid or aspirin is formed as a major product and acetic acid as minor product.

(b) C2H5ONa → C2H5O– + Na+
C2H5O– being a strong base. Moreover, in tert-butyl chloride, the a-carbon contains three alkyl group attached with it. Therefore, the molecule experience high steric hindrance. Both the factors combinedly lead to the elimination reaction. Hence, alkene will be formed.

2-Methylpropene will be formed.
(c) On treating phenol with chloroform in the presence of sodium hydroxide, a -CHO group is introduced at ortho position of benzene ring. This reaction is known as Reimer – Tiemann reaction.

o-Hydroxybenzaldehyde (or salicylaldehyde) will be formed.
Question 27.
Using Valence bond theory, explain the following in relation to the paramagnetic complex [Mn(CN)6]3-. [3]
(a) type of hybridization
(b) magnetic moment value
(c) type of complex – inner, outer orbital complex
Answer:
Oxidation state of Mn is x + 6(-1) = – 3
x = +3
Electronic configuration of Mn (in ground state) = [Ar]3d54s²
Electronic configuration of Mn3+ (in excited state) = [Ar]3d4

Six pairs of electrons, one from each CN molecule, occupy the six hybrid orbitals. Thus, the complex has octahedral geometry and is paramagnetic because of the absence of unpaired electron. In the formation of this complex, since the inner d-orbital (3d) is used in hybridisation, the complex, [Mn(CN)6]3- is called an inner orbital or low spin or spin paired complex.
Type of hybridization – d²sp3
Magnetic moment value – \(\sqrt{n(n+2)}=\sqrt{2(2+2)}\) = 2.87 BM
(n = number of unpaired electrons)
Question 28.
Answer the following questions: [3]
(a) State Henry’s law and explain why are the tanks used by scuba divers filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen)?
(b) Assume that argon exerts a partial pressure of 6 bar. Calculate the solubility of argon gas in water. (Given Henry’s law constant for argon dissolved in water, KH = 40kbar)
Answer:
(a) Henry’s law states that: ”The partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution”.
Scuba divers must cope with high concentrations of dissolved gases while breathing air at high pressure under water. Increased pressure, increases the solubility of atmospheric gases in blood. When the divers come towards surface, the pressure gradually decreases.
This releases the dissolved gases and leads to the formation of bubbles of nitrogen in the blood. To avoid bends, as well as, the toxic effects of high concentrations of nitrogen in the blood, the tanks used by scuba divers are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
(b) According to henry’s law :
P = KHX
Mole fraction of argon in water x = p/KH
= \(\frac { 6 }{ 40 }\) x 10³ = 1.5 x 10–
Question 29.
Give reasons for any three of the following observations: [3]
(a) Aniline is acetylated before nitration reaction.
(b) pKb of aniline is lower than the m-nitroaniline.
(c) Primary amine on treatment with benzenesulphonyl chloride forms a product which is soluble in NaOH however secondary amine gives product which is insoluble in NaOH.
(d) Aniline does not react with methyl chloride in the presence of anhydrous AlCl3 catalyst.
Answer:
(a) Direct nitration of aniline yields tarry oxidation products in addition to the nitro derivatives. Moreover, in the strong acidic medium, aniline is protonated to form the anilinium ion which is meta directing. That is why besides the ortho and para derivatives, significant amount of meta derivative is also formed. However, by protecting the -NH2 group by acetylation reaction with acetic anhydride, the nitration reaction can be controlled and the p-nitro derivative can be obtained as the major product.
(b) The presence of electron withdrawing groups like -NO2, -SO3H, -COOH, -X decreases the basic strength of the aniline. Since, pKb value is inversely proportional to basic strength. pKb of aniline is lower than the m-nitro aniline.The basic strength of aniline is more that m-nitroaniline.
(c) Benzenesulphonyl chloride (C6H5SO2Cl), which is also known as Hinsberg’s reagent, reacts with primary and secondary amines to form sulphonamides.
In the case of N-ethylbenzenesulphonyl amide is formed, the hydrogen attached to nitrogen in sulphonamide is strongly acidic due to the presence of strong electron withdrawing sulphonyl group. Hence, it is soluble in alkali.
While in case of secondary amines they not contain any hydrogen atom attached to nitrogen atom, it is not acidic and hence insoluble in alkali and tertiary amines do not react with benzenesulphonyl chloride.
(d) Aniline does not react with methylchloride in the presence of A1C13 catalyst, because of the formation of salt with aluminium chloride, the Lewis acid, which is used as a catalyst. Due to this, nitrogen of aniline acquires positive charge and hence acts as a strong deactivating group for further reaction.
![]()
Question 30.
(a) Identify the major product formed when 2-cy clohexylchloroethane undergoes a dehy drohalogenation reaction. Name the reagent which is used to carry out the reaction. [3]
(b) Why are haloalkanes more reactive towards nucleophilic substitution reactions than haloarenes and vinylic halides?
OR
(a) Name the possible alkenes which will yield 1-chloro-l-methylcyclohexane on their reaction with HCl. Write the reactions involved.
(b) Allyl chloride is hydrolysed more readily than n-propyl chloride. Why?
Answer:
(a) The given reaction follows Saytzeff’s rule i.e., highly substituted alkene is major product. When 2-cyclohexylchloroethane is allowed to react with strong base like ethanolic KOH, it undergoes elimination reaction (dehydrohalogenation reaction) to form 1- cyclohexylethene.

(b) Haloalkanes are more reactive than haloarenes and vinylic halides because of the following reasons:
(i) Resonance effect: C-Cl bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in haloarene is more difficult than haloalkane.
(ii) Difference in hybridisation of carbon atom in C-X bond: In haloalkane, the carbon atom attached to halogen is sp³-hybridised while in case of haloarene, the carbon atom attached to halogen is sp²-hybridised. Since it is difficult to break a shorter bond than a longer bond, therefore, haloarenes are less reactive than haloalkanes towards nucleophilic substitution reaction.
(iii) Instability of phenyl cation: In case of haloarenes, the phenyl cation formed as a result of self-ionisation will not be stabilised by resonance and therefore, SN1 mechanism is ruled out.
(iv) Repulsion: Due to the possible repulsion, it is less likely for the electron rich nucleophile to approach electron rich arenes.
(a) Methylenecy clohexane:

(b) Allyl chloride has high reactivity because the carbocation formed by hydrolysis is stabilised by resonance, whereas n-propyl chloride has no such carbocation stabilisation.
However, because n-propyl chloride does not undergo ionisation to produce n-propyl carbocation, allyl chloride is more easily hydrolyzed than n-propyl chloride.
Section – D
The following questions are case-based questions. Each question has an internal choice and carries 4 (1+1+2) marks each. Read the passage carefully and answer the questions that follow.
Question 31.
Strengthening the Foundation: Chargaff Formulates His “Rules” [3]
Many people believe that James Watson and Francis Crick discovered DNA in the 1950s. In reality, this is not the case. Rather, DNA was first identified in the late 1860s by Swiss chemist Friedrich Miescher. Then, in the decades following Miescher’s discovery, other scientists-notably, Phoebus Levene and Erwin Chargaff-carried out a series of research efforts that revealed additional details about the DNA molecule, including its primary chemical components and the ways in which they joined with one another.
Without the scientific foundation provided by these pioneers, Watson and Crick may never have reached their groundbreaking conclusion of 1953: that the DNA molecule exists in the form of a three-dimensional double helix.
Chargaff, an Austrian biochemist, as his first step in this DNA research, set out to see whether there were any differences in DNA among different species. After developing a new paper chromatography method for separating and identifying small amounts of organic material, Chargaff reached two major conclusions:
(i) the nucleotide composition of DNA varies among species.
(ii) Almost all DNA, no matter what organism or tissue type it comes from maintains certain properties, even as its composition varies. In particular, the amount of adenine (A) is similar to the amount of thymine (T), and the amount of guanine (G) approximates the amount of cytosine (C). In other words, the total amount of purines (A + G) and the total amount of pyrimidines (C + T) are usually nearly equal. This conclusion is now known as “Chargaff’s rule.”
Chargaff s rule is not obeyed in some viruses. These either have single- stranded DNA or RNA as their genetic material.
Answer the following questions:
(a) A segment of DNA has 100 adenine and 150 cytosine bases. What is the total number of nucleotides present in this segment of DNA?
(b) A sample of hair and blood was found at two sites. Scientists claim that the samples belong to same species. How did the scientists arrive at this conclusion?
(c) The sample of a virus was tested and it was found to contain 20% adenine, 20% thymine, 20 % guanine and the rest cytosine. Is the genetic material of this virus (a) DNA- double helix (b) DNA- single helix (c) RNA? What do you infer from this data?
OR
How can Chargaff’s rule be used to infer that the genetic material of an organism is double-helix or single-helix?
Answer:
(a) In a segment of DNA, number of Adenine = 100.
So the number of thymine =100.
And number of cytosine bases = 150.
So the number of guanine will be = 150
Therefore, Total nucleotides = 100 + 100 + 150 + 150 = 500
(b) Scientist studied the nucleotide composition of DNA. It was the same so they concluded that the samples belong to same species.
(c) Percentage of Adenine = Thymine = 20%
But the percentage of Guanine is not equal to Cytosine. So, double helix is ruled out. The bases pairs are ATGC and not AUGC so it is not RNA. The virus is a single helix DNA virus.
OR
According to Chargaff’s rule, all double helix DNA will have the same amount of Adenine and thymine as well as cytosine will be same amount as guanine. If this is not the case then the helix is single stranded.
![]()
Question 32.
Henna is investigating the melting point of different salt solutions.
She makes a salt solution using 10 mL of water with a known mass of NaCl salt.
She puts the salt solution into a freezer and leaves it to freeze.
She takes the frozen salt solution out of the freezer and measures the temperature when the frozen salt solution melts.
She repeats each experiment.
| S. No. | Mass of the salt used in g | Melting point in 0C | |
| Readings Set 1 | Reading Set 2 | ||
| 1. | 0.3 | -1.9 | -1.9 |
| 2. | 0.4 | -2.5 | -2.6 |
| 3. | 0.5 | -3.0 | -5.5 |
| 4. | 0.6 | -3.8 | -3.8 |
| 5. | 0.8 | -5.1 | -5.0 |
| 6. | 1.0 | -6.4 | -6.3 |
Assuming the melting point of pure water as 0°C, answer the following questions: [3]
(a) One temperature in the second set of results does not fit the pattern. Which temperature is that? Justify your answer.
(b) Why did Henna collect two sets of results?
(c) In place of NaCl, if Henna had used glucose, what would have been the melting point of the solution with 0.6 g glucose in it?
OR
What is the predicted melting point if 1.2 g of salt is added to 10 mL of water? Justify your answer.
Answer:
(a) For a pure liquid, The melting point of ice is the freezing point of water Using the depression in freezing point property in this case.
3rd reading for 0.5 g, there has to be an increase in depression of freezing point and therefore decrease in freezing point. So, also decrease in melting point when amount of salt is increased but the trend is not followed on this case.
(b) By collecting two sets of readings, help to avoid error in data collection and give more objective data.
(c) Expression for depression in freezing point – ∆Tf = iKfm
For glucose molecule,
∆Tf (glucose) = 1 x Kf x \(\frac{0.6 \times 1000}{180 \times 10}\)
For NaCl molecule,
∆Tf(Nacl) = 2 x Kf x \(\frac{0.6 \times 1000}{58.5 \times 10}\)
3.8 = 2 x Kf x \(\frac{0.6 \times 1000}{58.5 \times 10}\)
Divide equation (i) by (ii),
\(\frac{\left.\Delta \mathrm{T}_f \text { (glucose }\right)}{3.8}=\frac{58.5}{2 \times 180}\)
∆Tf(glucose) = 0.62
Freezing point or Melting point = – 0.62°C
OR
Since, depression in freezing point is directly proportional to molality (mass of solute when the amount of solvent remains same).
0.3 g depression is 1.9°C
0.6 g depression is 3.8°C
1.2 g depression will be 3.8 x 2 = 7.6°C
Section – E
The following questions are long answer type and carry 5 marks each. Two questions have an internal choice.
Question 33.
(a) Why does the cell voltage of a mercury cell remain constant during its lifetime? [5]
(b) Write the reaction occurring at anode and cathode and the products of electrolysis of aq KCl.
(c) What is the pH of HC1 solution when the hydrogen gas electrode shows a potential of -0.59 V at standard temperature and pressure?
OR
(a) Molar conductivity of substance “A” is 5.9 x 10³ S/m and “B” is 1 x 10-16 S/m. Which of the two is most likely to be copper metal and why?
(b) What is the quantity of electricity in Coulombs required to produce 4.8 g of Mg from molten MgCl2? How much Ca will be produced if the same amount of electricity was passed through molten CaCl2? (Atomic mass of Mg = 24 u, atomic mass of Ca = 40 u).
(c) What is the standard free energy change for the following reaction at room temperature? Is the reaction spontaneous?
Sn(s) + 2CU2+ (aq) a Sn2+ (aq) + 2Cu+ (s)
Answer:
(a) In a mercury cell,
Anode: Zinc – mercury amalgam Cathode: A paste of HgO and carbon Electrolyte: A paste of KOH and ZnO
Anode: Zn(Hg) + 2OH– → ZnO(s) + H2O + 2e–
Cathode: HgO + H2O + 2e– → Hg(l) + 2OH–
The overall reaction is represented by Zn(Hg) + HgO(s) → ZnO(s) + Hg(l)
The cell potential is approximately 1.35 V and remains constant during its life as the overall reaction does not involve any ion in solution whose concentration can change during its life time.
(b) Electrolysis of aqueous KC1,
KCl(aq) → K+(aq) + Cl–(aq)
Cathode: H2O(l) + e– → 1/2 H2(g) + OH–(aq)
Anode: Cl–(aq) → 1/2Cl2 (aq) + e–
Net reaction: KCl(aq) + H2O(l) → K+(aq) + OH–(aq) + 1/2 H2(g) + 1/2 Cl2(g)
Hence, Hydrogen gas will be released at cathode, chlorine gas at anode and KOH will remain in the solution as a byproduct.
(c) Electrode reaction: H+ + e– → 1/2 H2
Given, E\(\left(\mathrm{H}^{+} / \mathrm{H}_2\right)\) = – 0.59 V and we know that, \(\mathrm{E}_{\left(\mathrm{H}^{+} / \mathrm{H}_2\right)}^{\Upsilon}\) = 0 V
Applying Nemst equation,

OR
(a) Conductors are those substances that conducts electricity. These substances have high value of conductivity. Out of the two given substances, A has a higher values of conductivity is 5.9 x 103 S/m). As metals are good conductors of electricity and copper is a metal. Hence, substance A should be a metal i.e., copper.
(b) In molten MgCl2, production of metal magnesium takes place as follows:
Mg2+ + 2e– → Mg
1 mole of magnesium ions gains two moles of electrons or 2F to form 1 mole of Mg
Therefore, 24 g Mg will requires 2 F electricity
And 4.8 g Mg requires 2 x \(\frac { 4.8 }{ 2.4 }\) = 0.4 F
= 0.4 x 96500 = 38600C
For CaCl2,
Ca2+ + 2e– → Ca
2 F electricity is required to produce 1 mole = 40 g Ca 0
0.4 F electricity will produce 8 g Ca
(c)

Question 34.
A hydrocarbon (A) with molecular formula C5H10 on ozonolysis gives two products (B) and ( C). Both (B) and (C) give a yellow precipitate when heated with iodine in presence of NaOH while only (B) give a silver mirror on reaction with Tollen’s reagent. [5]
(a) Identify (A), (B) and (C).
(b) Write the reaction of B with Tollen’s reagent.
(c) Write the equation for iodoform test for C.
(d) Write down the equation for aldol condensation reaction of B and C.
OR
An organic compound (A) with molecular formula C2Cl3O2H is obtained when (B) reacts with Red P and Cl2. The organic compound (B) can be obtained on the reaction of methyl magnesium chloride with dry ice followed by acid hydrolysis.
(a) Identify A and B
(b) Write down the reaction for the formation of A from B. What is this reaction called?
(c) Give any one method by which organic compound B can be prepared from its corresponding acid chloride.
(d) Which will be the more acidic compound (A) or (B)? Why?
(e) Write down the reaction to prepare methane from the compound (B).
Answer:
The hydrocarbon A with molecular formula similar to alkene or cycloalkane.
But since, A undergoes ozonolysis reaction, it must be an alkene.
Compound B and C must be methylated aldehyde or ketones as both of them shown iodoform reaction. Now, since compound B shows silver mirror test, it must be methylated aldehyde and C must be methylated ketone.

OR
According to the question, the compound B undergoes Hell Volhard Zelinsky reaction, when allowed to reacts with Red P and Cl2 gas. Thus compound B must be a carboxylic acid and and compound A must be a-halocarboxylic acids.
(a) Compound B – CH3COOH and Compound A – CCl3COOH
![]()
This reaction is commonly called as Hell Volhard Zelinsky reaction.
(c) Acid chlorides when hydrolysed with water give carboxylic acids.
![]()
(d) Electron withdrawing groups increase the acidity of carboxylic acids by stabilising the conjugate base through delocalisation of the negative charge by inductive and/or resonance effects.
Cl being electron withdrawing group, presence of 3C1 atoms in compound A makes it more acidic as compared to B.
(e) Compound B undergoes decarboxylation reaction when treated with CaO in the presence of NaOH to form the corresponding alkane.
![]()
Question 35.
Answer the following: [5]
(a) Why are all copper halides known except that copper iodide?
(b) Why is the V°\(\left(\mathrm{V}^{3+} / \mathrm{V}^{2+}\right)\) value for vanadium comparatively low?
(c) Why HC1 should not be used for potassium permanganate titrations?
(d) Explain the observation, at the end of each period, there is a slight increase in the atomic radius of d block elements.
(e) What is the effect of pH on dichromate ion solution?
Answer:
(a) The iodide ion being a bulky ion, and strong reducing agent reduces copper iodide to cupric iodide.
2CuI2 → 2CuI + I2
Or the cupric ions oxidised the iodide ions into the molecular iodine and itself get reduced to cuprous ions. Hence, all copper halides known except that copper iodide.
(b) Vanadium having atomic number 23 and electronic configuration – [Ar]4s²3d³.
Electronic configuration of V+2 – [Ar]3r³.
It has half filled t2g configuration due to which it quite stable and hence V+3 readily reduces to V+2.
(c) It reacts with the titration indicator potassium permanganate (KMnO4) and oxidises it resulting into the release of chlorine gas. At the end of the process, the final value of the indicator used can differ greatly from the total value of the reaction. This will cause the entire experiment to be disrupted. In essence, in volumetric analysis, HC1 cannot be used to acidify KMnO4.
(d) Across the period, among d-block elements, the atomic size first decreases with increase in atomic number, then remains constant and then slightly increases.
At the end of each period among d-block elements, the atomic size slightly increases. It is because of the presence fully filled d-orbital, when pairing takes place,the e– – e– repulsion between the electrons increases.
(e) The chromates and dichromates are interconvertible in aqueous solution depending upon pH of the solution. Increasing the pH (in basic solution)of dichromate ions a colour change from orange to yellow is observed as dichromate ions change to chromate ions.